
A retrospective study of pediatric brain tumors from a tertiary care hospital in South China
Introduction
The management of central nervous system (CNS) tumors in children requires a concerted multidisciplinary approach involving radiologists, neurosurgeons, pathologists, and—depending on the tumor type—radiation oncologists and pediatric oncologists. Such requirements have limited the development of comprehensive pediatric neuro-oncology programs, particularly in countries with limited health care resources.
Although some studies suggest a lower incidence of CNS tumors in Chinese children (1), we estimate the annual number of brain tumors in the pediatric population to be around 10,000 cases per year (2). Despite this impressive number, information regarding management and outcome remains relatively limited (3-6), and the organization and standardization of care for pediatric brain tumors lag behind that of other childhood malignancies, such as leukemia (7). In this context, it is crucial to analyze the characteristics of current pediatric neuro-oncology practices in large Chinese institutions to identify the steps required to implement a coordinated national approach for this group of patients.
Shenzhen is a large city in South China with over 17 million people. Shenzhen Children’s Hospital (SZCH) was established in 1997 and is the only tertiary children’s hospital in Shenzhen at present. It has a dedicated neurosurgery department and a hematology-oncology unit but no radiotherapy facility. Here we report the characteristics, management, and outcome of pediatric patients with CNS tumors seen at SZCH between 2015 and 2018. We present the following article in accordance with the STROBE reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-92/rc).
Methods
Clinical data and statistical analyses
A retrospective study was conducted of brain tumor cases [International Classification of Diseases (ICD)-10 category C71] between 0 and 18 years of age diagnosed in SZCH from January 1, 2015, to December 31, 2018. The date of 2015 was chosen because it coincides with the initiation of the Sanming Project, a 5-year collaboration between SZCH and the Division of Hematology/Oncology of The Hospital for Sick Children, Toronto. Most clinical medical records were received from the Department of Pediatric Neurosurgery, whilst a few cases were retrieved from the Department of Hematology and Oncology. Initially, all biopsies were classified using the WHO 2007 classification (8), and subsequently, the WHO 2016 (9) classification was utilized.
Clinical data were collected on demographics, diagnosis, treatment, and follow-up information. We analyzed the annual number of newly diagnosed cases each year, as well as their distribution according to pathology. To better understand the trajectory of management, we collected information on the sites where patients were diagnosed and subsequently treated. Follow-up data were carefully collected and measured by last time seen or phone call to the family.
The age values are described as the median (range), and count data are described by frequency and rate. Overall survival (OS) and event-free survival (EFS) were analyzed using Kaplan-Meier curves. OS was defined as the time elapsed between the date of diagnosis and that of the last follow-up or death by any cause. EFS was measured from the first day of diagnosis and was defined as survival without progression, relapse, or death from another cause. The statistical analysis was performed with SPSS software version 26.0 (IBM Corp., Armonk, NY, USA).
Compliance with ethical standards
This study was approved by the Institutional Review Board of Shenzhen Children’s Hospital (No. 2021065). The study was performed in compliance with the Declaration of Helsinki (as revised in 2013) and other relevant regulations. The informed consent was taken from all individual participants’ parents or legal guardians.
Results
Diagnosis
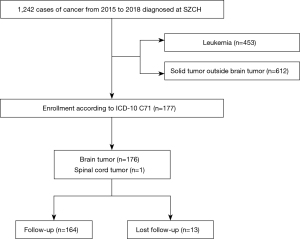
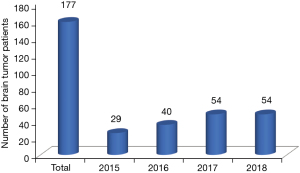
Out of 1,242 children with cancer seen during the time frame, 177 patients diagnosed with pediatric brain and spinal tumors (14.3%) were identified (Figure 1). The median age at diagnosis was 4.8 years (range, 3 months to 16 years). One hundred and seven patients (60.5%) were males, and 70 (39.5%) were females with a male to female ratio of 1.5:1. The number of patients increased steadily between 2015 and 2018 from 29 to 54, as shown in Figure 2. One hundred and fifty-five cases (87.6%) underwent a surgical biopsy or resection with a diagnosis based on pathological results. The remaining 22 patients were diagnosed based on radiological findings and clinical manifestations, leading to a consensus decision not to proceed to surgery. They included 12 brainstem tumors (diffuse intrinsic pontine glioma) and three cases of presumed germ cell tumors. The most common diagnoses were glioma [63 cases, 35.6%, including nine histologically confirmed high-grade gliomas (HGGs)], medulloblastoma (37 cases, 20.9%), and ependymoma (19 cases, 10.7%).
Therapy
Management
Table 1 shows the main types of treatment for brain tumors, which included surgery, chemotherapy, radiation, and high dose chemotherapy with autologous hematopoietic stem cell transplantation. Overall, 160 patients (90.4%) underwent surgery for resection, biopsy, and/or ventriculo-peritoneal shunt, which accounted for 151, 4, and 85 cases, respectively. Among the 85 shunts, 80 were combined with resections, and five had shunts only. Of the latter five patients, four had brain stem tumors, and one had a posterior fossa mass. The extent of resection for the most common entities [medulloblastoma, ependymoma, low-grade glioma (LGG), and HGG] is shown in Table 2. Ninety-five patients (53.6%) received chemotherapy, and 87 patients (49.2%) received radiotherapy.
Table 1
| Management | Number | Percentage |
|---|---|---|
| Surgery (and/or ventriculo-peritoneal shunt) | 160 | 90.4% (160/177) |
| Chemotherapy | 95 | 53.7% (95/177) |
| Radiotherapy | 87 | 49.2% (87/177) |
| Autologous peripheral stem cell transplantation | 5 | 2.8% (5/177) |
Table 2
| Tumor type | N | Gross total resection, n (%) | Subtotal/partial resection, n (%) | Biopsy, n (%) |
|---|---|---|---|---|
| Medulloblastoma | 37 | 26 (70.2) | 11 (29.7) | 0 (0.0) |
| Ependymoma | 19 | 15 (78.9) | 3 (15.8) | 1 (5.3) |
| LGG | 40 | 30 (75.0) | 8 (20.0) | 2 (5.0) |
| HGG | 9 | 3 (33.3) | 5 (55.6) | 1 (11.1) |
LGG, low-grade glioma; HGG, high-grade glioma.
Table 3 provides details on the various approaches used to manage patients. The most common treatment combination was surgery with chemotherapy and radiotherapy, accounting for 31.1% of the entire population. Other treatment combinations were as follows: surgery and observation, 27.7%; surgery and chemotherapy, 10.7%; surgery and radiotherapy, 7.3%; chemotherapy and radiotherapy, 5.6%; surgery, chemotherapy, and high dose chemotherapy with autologous peripheral stem cell transplantation with or without radiotherapy, 1.7%.
Table 3
| Management | Number | Percentage |
|---|---|---|
| Surgery and observation | 49 | 27.7% (49/177) |
| Surgery and chemotherapy | 19 | 10.7% (19/177) |
| Surgery and radiotherapy | 13 | 7.3% (13/177) |
| Surgery, chemotherapy, and radiotherapy | 55 | 31.1% (55/177) |
| Chemotherapy and radiotherapy | 10 | 5.6% (10/177) |
| Surgery, chemotherapy, and autologous peripheral stem cell transplantation | 2 | 1.1% (2/177) |
| Surgery, chemotherapy, radiotherapy, and autologous peripheral stem cell transplantation | 3 | 1.7% (3/177) |
| Other* | 26 | 14.7% (26/177) |
*, includes chemotherapy only (3 cases), radiotherapy only (4 cases), observation only (3 cases), deaths during surgery (3 cases), lost to follow up and treatment abandonment (13 cases).
Location of treatment
Table 4 provides additional information on the distribution of treatments and their location. The percentage of patients who underwent surgery, either biopsy or resection, in SZCH was 89.7% (139/155), while 10.3% had their initial surgery outside SZCH (16/155). For chemotherapy, 71.6% of patients were treated at SZCH, and the remaining 28.4% received chemotherapy at different institutions. Significantly, five out of 68 patients received chemotherapy in the Department of Hematology and Oncology, while 63 patients were treated in the Department of Neurosurgery at SZCH. In addition, five patients who received their initial chemotherapy in the Department of Neurosurgery were referred to the Bone Marrow Transplant (BMT) Unit for high-dose chemotherapy with autologous stem cell transplant. Due to the lack of a dedicated pediatric radiotherapy facility at SZCH, most patients (82/87) who needed radiation treatment were transferred to qualified institutions in China, including Beijing, Shanghai, Guangzhou, and Changsha. Only five patients were treated at a radiation facility in Shenzhen. The nearest pediatric radiation facility to Shenzhen is in Guangzhou, which is 140 kilometers away, and the farthest one is in Beijing, which is 2,200 kilometers away. Overall, 43.7% (38/87) of the patients received radiation in Guangzhou.
Table 4
| Management | Total | Sites | Number | Percentage |
|---|---|---|---|---|
| Surgery | 155 | In SZCH | 139 | 89.7% (139/155) |
| Outside Shenzhen | 16 | 10.3% (16/155) | ||
| Chemotherapy | 95 | In SZCH | 68 | 71.6% (68/95) |
| Department of Neurosurgery | 63 | 92.6% (63/68) | ||
| Department of Hematology and Oncology | 5 | 7.3% (5/68) | ||
| BMT Unit† | 5 | 7.3% (5/68) | ||
| Outside Shenzhen | 27 | 28.4% (27/95) | ||
| Radiotherapy | 87 | In Shenzhen | 5 | 5.7% (5/87) |
| Outside Shenzhen | 82 | 94.3% (82/87) | ||
| Beijing | 3 | 3.4% (3/87) | ||
| Shanghai | 2 | 2.2% (2/87) | ||
| Guangzhou | 38 | 43.7% (38/87) | ||
| Other cities | 2 | 2.2% (2/87) | ||
| Unknown | 37 | 42.5% (37/87) |
†, initial chemotherapy in Department of Neurosurgery and high-dose chemotherapy in BMT Unit of Department of Hematology and Oncology. SZCH, Shenzhen Children’s Hospital; BMT, Bone Marrow Transplant.
Follow-up
The follow-up time was from the first diagnosis until December 31, 2020. All patients were followed up from 1 day to 70.7 months (median, 34.6 months). Thirteen patients, accounting for 7.3% (13/177), were lost to follow-up after they were referred for radiotherapy to other hospitals (7/13) or abandoned treatment (6/13). Due to the limited information in the records on long-term impairments, quality of life, or outcomes, collecting data on long-term side effects was not possible.
Histological types and outcomes
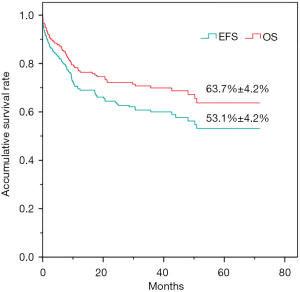
Fifty-four patients (30.5%) succumbed. The leading cause of death was disease progression, which accounted for 90.7% (49/54) of all deaths. Glioma, medulloblastoma, ependymoma, and other CNS embryonal tumors accounted for 17, 10, 6, and 5 cases, respectively. The percentage of surviving patients for these four types was 65.1%, 70.2%, 58%, and 40.0%, respectively (Table 5). The 5-year OS rate of the entire cohort of 177 patients was 63.7%±4.2%, while the EFS was 53.1%±4.2% (Figure 3).
Table 5
| Histological types | Number (%) | Death from progression | Death from other causes | Patients lost to FU | Alive with FU data (%) |
|---|---|---|---|---|---|
| Glioma | 63 (35.6) | 17 | 3 (intratumoral bleed; postoperative herniation; septic shock) | 2 (abandonment) | 41 (65.1) |
| LGG | 40 | ||||
| HGG | 9 | ||||
| Unknown | 14 | ||||
| Medulloblastoma | 37 (20.9) | 10 | 1 (TTOH) | 26 (70.2) | |
| Ependymoma | 19 (10.7) | 6 | 2 (TTOH) | 11 (58.0) | |
| Other CNS embryonal tumors | 10 (5.6) | 5 | 1 (intratumoral bleed) | 4 (40.0) | |
| Craniopharyngioma | 9 (5.1) | 9 (100.0) | |||
| Atypical teratoid/rhabdoid tumor | 9 (5.1) | 3 | 1 (intraoperative hemorrhage) | 2 (TTOH) | 3 (33.3) |
| Germ cell tumor | 8 (4.5) | 2 | 2 (TTOH) | 4 (50.0) | |
| Choroid plexus tumors | 6 (3.4) | 1 | 2 (abandonment) | 3 (50.0) | |
| Dysembryoplastic neuroepithelial tumor | 4 (2.3) | 4 (100.0) | |||
| Melanoma | 2 (1.1) | 2 | 0 (0.0) | ||
| Meningioma | 2 (1.1) | 1 (abandonment) | 1 (50.0) | ||
| Pineal parenchymal tumor | 1 (0.6) | 1 (100.0) | |||
| Neurocytoma | 1 (0.6) | 1 (100.0) | |||
| Ewing sarcoma-like tumor | 1 (0.6) | 1 (100.0) | |||
| Unknown | 5 (2.8) | 3 | 1 (abandonment) | 1 (20.0) | |
| Total | 177 | 49 | 5 | 13 | 110 |
LGG, low-grade glioma; HGG, high-grade glioma; CNS, central nervous system; FU, follow-up; TTOH, transferred to other hospital.
Discussion
This review of 177 pediatric CNS tumor patients seen at SZCH over a 4-year period illustrates several challenges associated with the management of this population in South China. While the number of patients admitted has steadily increased over the period of time, the annual number of diagnoses does not match the expected incidence in this large city. With an official population of 17.5 million people, which is likely an underestimation due to a large number of unregistered migrants and a very young population compared with other parts of China, the 177 patients seen represent only a subset of CNS tumor patients. In the absence of a provincial and national cancer registry, it is impossible to have a clear idea of the epidemiology of pediatric CNS tumors and a good understanding of the management and the pattern of referral of these patients. China is the largest developing country without a national brain tumor registry. It remains challenging to systematically collect data on patients with brain tumors in China and difficult to perform an in-depth analysis on the status of patients with brain tumors, their management, and outcomes in the Chinese population. A government-funded national data collection will be conducted continuously from February 1, 2019, to January 31, 2024. The first results of this effort are expected in 2024 (10).
The distribution of histology in this study is unusual in the context of China. The proportion of medulloblastoma patients is slightly higher than in a recent epidemiological study conducted in Hong Kong, a city close to Shenzhen (1). The most striking difference is the low proportion of patients with germ cell tumors, a group that accounts for only 4% of the cases seen. In most literature reports from China and Eastern Asia, the proportion of patients with germ cell tumors exceeds that of patients with medulloblastoma (11,12). The reason for this difference is unknown. However, it suggests that the referral pattern to SZCH is selective and that patients with a presumed diagnosis of germ cell tumor may be referred to other hospitals where radiation facilities are available.
LGG, which has been listed in the six diseases targeted by the WHO Global Initiative for Childhood Cancer in September 2018, was the largest group accounting for one-fifth to one-fourth of patients in our study. These patients were followed up by neurosurgeons after surgery in the clinic and treated in the neurosurgery unit when chemotherapy was considered. However, with the major advances in understanding the molecular biology of this condition that have happened during the last decade, the management of this condition is becoming increasingly complex because of the recent availability of targeted therapies. These advances are unlikely to benefit LGG patients without multidisciplinary cooperation between neurosurgery, radiology, pathology, radiotherapy, oncology, ophthalmology, and rehabilitation. As the survival rate of LGG patients is usually excellent, efforts should be made to improve their quality of life and prevent long-term complications by optimal management and effective long-term follow-up care.
While the increase over this period of 4 years is an acknowledgment of the visibility of the neurosurgery program at SZCH, a large number of patients eventually received postoperative treatment at other centers. This is particularly the case for patients requiring additional radiotherapy treatment: 82 out of 87 patients were referred outside Shenzhen, mostly to Guangzhou, which is 140 km away. Similarly, but to a lesser extent, one-third of patients who received chemotherapy were treated outside SZCH. There are many risks associated with such fragmented care, including incomplete sharing of information, delays in transfers, and loss of follow-up. Ideally, it would be beneficial to collaborate with radiation centers within Shenzhen, as this would allow better interaction between professionals and reduce travel-related stress and expenditure for families. There are ongoing discussions with Shenzhen People’s Hospital and Shenzhen Cancer Institute regarding this opportunity. However, the main limitation is their limited experience in managing pediatric patients. There is an urgent need to develop multidisciplinary programs that can offer comprehensive care to these children, including rehabilitation and after-care follow-up. This study constitutes a baseline assessment of a still-developing experience. Although the number of patients has gradually increased, there is no evidence that referral patterns have changed during the study period. As far as outcomes are concerned, this work also represents a baseline. We hope that further follow-up will offer the opportunity to analyze the impact of ongoing coordinated efforts on outcomes.
Another characteristic concerns the administration of chemotherapy within the neurosurgery department. The large majority of patients (63/68) received chemotherapy in the neurosurgical unit. Such practice is common in China and other Asian countries. However, it is in contrast to current practices in Europe and North America, where chemotherapy administration is under the responsibility of trained pediatric oncologists and usually given in the pediatric oncology department by qualified pediatric oncology nurses. Cases diagnosed in the neurosurgery department were not discussed regularly, and it was unusual to invite oncologists to join the discussion. The reason for such practice is essentially historical. However, with the increasing complexity of protocols, the growing importance of molecular classifications to tailor treatments for childhood brain tumors (13), and the importance of considering all the needs of the patients, including rehabilitation (14) and palliation (15), it is critical to clarify the role of every specialty in the management of this complex care population. One particularity of this study is the referral of a subset of patients to the BMT Unit. The selection of indications was discussed in the context of an ongoing collaboration between SZCH and The Hospital for Sick Children in Toronto and only concerned patients who were considered high risk, with a potential benefit of an intensification of therapy. It is interesting to note that all five patients were alive at their last follow-up, including two infants with medulloblastoma and pineoblastoma, respectively, who did not receive any radiation treatment as part of their management.
Treatment abandonment is always a concern in low- and middle-income countries (LMIC) (16). In the current study, six patients abandoned treatment. This figure is much lower than the 31% rate of abandonment reported by Seah et al. in a cohort of patients with medulloblastoma (16). The main reasons for abandonment in Seah’s series were misunderstandings about medulloblastoma and financial or transportation difficulties. We did not collect the specific reasons for abandonment in our series. However, most patients had either advanced disease or diseases with a poor prognosis.
Additionally, we did not collect specific information on the chemo- and radiotherapeutic management of the patients in this study, as there were several issues in doing so. The exact dates, doses, and radiotherapy volumes were not available for most patients treated outside Shenzhen. Similarly, information on the chemotherapy protocols used, dose adjustments, and side effects were lacking for most patients treated at outside institutions. At SZCH, guidelines have been developed for some diagnoses, particularly for medulloblastoma. However, no patient in this study has been treated in the context of a clinical trial. With the emergence of national networks through the Chinese Children’s Cancer Group (CCCG) and the Chinese Society of Neuro-Oncology, it is critical to develop protocols and/or national guidelines that allow consistency in managing this population across different centers. In this context, the National Health Commission of the People’s Republic of China has recently released notifications for the management of pediatric brain tumors, including glioma, medulloblastoma, craniopharyngioma, ependymoma, and CNS germ cell tumors (http://www.nhc.gov.cn/yzygj/s7659/202105/3c18fec8a37d452b82fe93e2bcf3ec1e.shtml; guidelines are in Chinese only; accessed August 17, 2021).
Similarly, we were unable to collect detailed follow-up information. Studies on long-term follow-up have identified pediatric patients with brain tumors as a high-risk population in regard to long-term sequelae (17,18) and poor social outcomes (19,20). Therefore, there is an urgent need to develop after-care programs that focus on the many complications associated with the diagnosis and management of this fragile population. The CCCG is actively working on implementing strategies to facilitate the follow-up of long-term survivors (19).
Conclusions
In this experience, there has been a gradual increase of pediatric neuro-oncological patients seen at SZCH, a tertiary institution in South China. Options for adjuvant treatment have been implemented, including chemotherapy, radiation, and, more recently, high dose chemotherapy with autologous stem cell rescue. As in other resource-limited countries, our survival rate is still lower than that reported in European and North American studies, and it is essential to strengthen the multidisciplinary collaboration for holistic management with coordinated care, including long-term follow-up.
Acknowledgments
We are grateful to the SickKids team involved in collaborating with SZCH, who freely shared their experiences and allowed us to conduct a meaningful study.
Funding: This work was supported by the Sanming Project of Medicine in Shenzhen (No. SZSM201512033) and the Guangdong Medical Science and Technology Research Project (No. A2020101).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ibrahim Qaddoumi, Anthony Liu and Chenchen Sun) for the series “Pediatric CNS Tumors in China” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-92/rc
Data Sharing Statement: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-92/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-92/coif). The series “Pediatric CNS Tumors in China” was commissioned by the editorial office without any funding or sponsorship. EB, FW, and HM report that the research was supported by the Sanming Project of Medicine in Shenzhen. The Sanming Project was established by the Shenzhen Municipal Government to support the collaboration between Shenzhen Children’s Hospital and the Division of Hematology/Oncology of the Hospital for Sick Children for 5 years from 2016 to 2020. HM also reports that the research was supported by the Guangdong Medical Science and Technology Research Project. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Shenzhen Children’s Hospital (No. 2021065). The study was performed in compliance with the Declaration of Helsinki (as revised in 2013) and other relevant regulations. The informed consent was taken from all individual participants’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu APY, Liu Q, Shing MMK, et al. Incidence and Outcomes of CNS Tumors in Chinese Children: Comparative Analysis With the Surveillance, Epidemiology, and End Results Program. JCO Glob Oncol 2020;6:704-21. [Crossref] [PubMed]
- Jiang T, Tang GF, Lin Y, et al. Prevalence estimates for primary brain tumors in China: a multi-center cross-sectional study. Chin Med J (Engl) 2011;124:2578-83. [PubMed]
- Liu AP, Shing MM, Yuen HL, et al. Central nervous system tumors in chinese children under the age of 3: a population study. J Pediatr Hematol Oncol 2015;37:94-103. [Crossref] [PubMed]
- Wang C, Yuan XJ, Jiang MW, et al. Clinical characteristics and abandonment and outcome of treatment in 67 Chinese children with medulloblastoma. J Neurosurg Pediatr 2016;17:49-56. [Crossref] [PubMed]
- Ji J, Luo Z, Chen Y, et al. Characteristics and trends of childhood cancer in Pudong, China, 2002-2015. BMC Public Health 2020;20:1430. [Crossref] [PubMed]
- Zhang ZY, Xu J, Ren Y, et al. Medulloblastoma in China: clinicopathologic analyses of SHH, WNT, and non-SHH/WNT molecular subgroups reveal different therapeutic responses to adjuvant chemotherapy. PLoS One 2014;9:e99490. [Crossref] [PubMed]
- Chen SL, Zhang H, Gale RP, et al. Toward the Cure of Acute Lymphoblastic Leukemia in Children in China. JCO Glob Oncol 2021;7:1176-86. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Zhang L, Jia W, Ji N, et al. Construction of the National Brain Tumor Registry of China for better management and more efficient use of data: a protocol. BMJ Open 2021;11:e040055. [Crossref] [PubMed]
- Dho YS, Jung KW, Ha J, et al. An Updated Nationwide Epidemiology of Primary Brain Tumors in Republic of Korea, 2013. Brain Tumor Res Treat 2017;5:16-23. [Crossref] [PubMed]
- Makino K, Nakamura H, Yano S, et al. Population-based epidemiological study of primary intracranial tumors in childhood. Childs Nerv Syst 2010;26:1029-34. [Crossref] [PubMed]
- Hanz SZ, Adeuyan O, Lieberman G, et al. Clinical trials using molecular stratification of pediatric brain tumors. Transl Pediatr 2020;9:144-56. [Crossref] [PubMed]
- Runco DV, Yoon L, Grooss SA, et al. Nutrition & Exercise Interventions in Pediatric Patients with Brain Tumors: A Narrative Review. J Natl Cancer Inst Monogr 2019;2019:163-8. [Crossref] [PubMed]
- Massie AM, Ebelhar J, Allen KE, et al. Defining and timing of palliative opportunities in children with central nervous system tumors. Neurooncol Pract 2021;8:451-9. [Crossref] [PubMed]
- Seah T, Zhang C, Halbert J, et al. The magnitude and predictors of therapy abandonment in pediatric central nervous system tumors in low- and middle-income countries: Systematic review and meta-analysis. Pediatr Blood Cancer 2019;66:e27692. [Crossref] [PubMed]
- Aukema EJ, Schouten-van Meeteren AY, Last BF, et al. Childhood brain tumor survivors at risk for impaired health-related quality of life. J Pediatr Hematol Oncol 2013;35:603-9. [Crossref] [PubMed]
- Varedi M, Lu L, Phillips NS, et al. Balance impairment in survivors of pediatric brain cancers: risk factors and associated physical limitations. J Cancer Surviv 2021;15:311-24. [Crossref] [PubMed]
- Cheung YT, Zhang H, Cai J, et al. Identifying Priorities for Harmonizing Guidelines for the Long-Term Surveillance of Childhood Cancer Survivors in the Chinese Children Cancer Group (CCCG). JCO Glob Oncol 2021;7:261-76. [Crossref] [PubMed]
- Brinkman TM, Ness KK, Li Z, et al. Attainment of Functional and Social Independence in Adult Survivors of Pediatric CNS Tumors: A Report From the St Jude Lifetime Cohort Study. J Clin Oncol 2018;36:2762-9. [Crossref] [PubMed]
Cite this article as: Mai H, Wang J, Cai L, Song J, Gan Y, Zhang X, Li Q, Ye H, Liu Y, Wen F, Chen Q, Bouffet E. A retrospective study of pediatric brain tumors from a tertiary care hospital in South China. Pediatr Med 2022;5:33.

