
Bronchopulmonary dysplasia with focus on early prediction and treatment: a narrative review
Introduction
Bronchopulmonary dysplasia (BPD) is a pulmonary disease seen in very preterm infants. In extremely premature infants with gestational age (GA) <26 weeks the incidence is 56% and in infants with GA ≥31 weeks the incidence decreases to four percent (1). BPD has a high mortality and morbidity as well as high treatment costs, particularly as the severity of disease increases. It may last for months and sequelae often last for years (2) and remains the most frequent complication of extreme preterm birth (3,4). BPD is multifactorial and not fully understood because several different insults both pre- and postnatal may contribute to the evolution and progression of BPD (5,6). Furthermore, some infants may be at risk because of a genetic predisposition (7). It is now accepted by most researchers that BPD is initiated by intrauterine infection either as chorioamnionitis (4,8-11) or as a silent infection where the microorganisms invade the fetal lungs (10). The same infection may initiate premature rupture of the membranes and preterm labour (4,9,10) associated with the birth of a premature infant. The low-virulence microorganisms have often been identified as mycoplasma or Ureaplasma species (12-14) causing active inflammation for weeks or months postnatally. BPD is often considered as an inflammatory disease and in addition to the initial inflammatory response, it is likely that an aberrant long lasting repair response is present in many cases (4). It is this persistent inflammation that results in abnormal development of the preterm lungs resulting in BPD.
There are no curative treatments neither in the early or late phases of BPD and further research about the epidemiology, pathobiology and pathophysiology is needed to improve the outcome through trials with early treatments. This review focuses on symptomatic treatment in the early phase of the disease which may improve outcome and, on the potential to predict the disease early with the possibility of interventions to ameliorate the course of BPD.
The article is divided in the following segments: “Introduction”, “Methods”, “Background”, “Clinical treatment in the early phases of BPD”, “Early prediction of BPD”, “Potential early therapies and prevention in at-risk babies” and “Conclusions”. Treatment of the fully developed BPD syndrome beyond day 28 and later will not be considered in this review. We present this article in according with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-98/rc).
Methods
The search strategy summary is described in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | The date of search was December 31, 2021 |
| Databases and other sources searched | We used the online databases PubMed and Medline. There have been no restrictions in the searched literature. Furthermore, data was hand search in Pediatrics, J Pediatr, Pediatric Research, Front Pediatr, Jama Pediatrics, N Engl J Med, Lancet, Arch Dis Child, Neonatology, Acta Paediatr, J Perinatol, Cochrane Database Syst Rev, J Exp Pharmacol, well-known Pediatric and Pharmacologic journals |
| Search terms used | The search terms were “Prediction of BPD” and “Early treatment of BPD” |
| Timeframe | Data were searched systematically up to December 31, 2021. The timeframe was January 1, 2011 to December 31, 2021 |
| Inclusion and exclusion criteria | The inclusion criteria were the same as described in search terms. No specific exclusion criteria. The language in the references was English. But there have been no language restrictions |
| Selection process | All authors conducted the selections especially the clinicians HV, CH, RR, ZKL, DS and HC |
BPD, bronchopulmonary dysplasia.
The literature about prediction of BPD and early treatment of BPD were searched online via the databases PubMed and Medline. Because very early prevention of BPD in at-risk babies has not been possible before the literature were then hand searched in pediatric and pharmacologic journals to evaluate and describe the potential for very early treatment and prevention of BPD.
Background
The term “bronchopulmonary dysplasia” was used for first time in 1967 by Northway (15). He used the term for the chronic lung injury seen in infants after aggressive mechanical ventilation. This old type of BPD is seldom seen today. So called ‘new BPD’ seen nowadays is normally preceded by increased alveolar-capillary permeability in the first 1–2 weeks after birth (16) with increasing need of oxygen and lung edema as early clinical findings within 10 days after birth. Radiographically, hazy ground-glass opacity within 12–24 hours (17) is suggestive of a preceding intrauterine infection as the triggering event of BPD. New BPD is also characterized by pulmonary vasculature hypoplasia and alveolar hypoplasia in very preterm infants (5). Cystic emphysema called Wilson-Mikity syndrome (18) was previously seen often. Today this condition has largely disappeared probably due to gentler ventilation. In Japan the Wilson-Mikity syndrome is still seen in about 13–14% of babies with BPD (19) and the condition in these patients seem to be linked to high leucocyte elastase and low α1-antitrypsin (20).
Definition of BPD
The Consensus verified BPD definition from the US National Institutes of Health (NIH) (21) is currently the most used. According to this definition, moderate BPD is diagnosed in infants born <32 weeks gestation needing supplemental oxygen for 28 days and at 36 weeks postmenstrual age (PMA) or at discharge. However, the definition of BPD is still a topic of debate. A concise and clearly defined consensus BPD definition, outlining risk severity, is in all cases beneficial for both clinical purposes and for research and with such a definition, methods to accurately predict BPD severity may help individualize early treatment therapies.
Clinical treatment in the early phases of BPD
Many of the current clinical treatments of BPD such as early nasal continuous positive airway pressure (NCPAP), surfactant, caffeine and optimal nutrition are necessary early therapies together with new early therapies in at-risk babies.
NCPAP and surfactant to avoid barotrauma and oxytrauma
Treatment with NCPAP early after birth vs. invasive mechanical ventilation (IMV) has a positive effect on respiratory distress syndrome (RDS) (22,23) and because of this also on BPD (24). The combined use of NCPAP and early, rescue surfactant with less invasive surfactant administration (LISA) technique (25,26) and the INSURE (INtubate SURfactant Extubation) method (27,28) are ideal in this context (29,30). However, only 80–85% of the infants with BPD had a diagnosis of RDS (31) reflecting that BPD is not a continuum of RDS but representing a different entity (31,32). Therefore, these treatments are not the only ideal early treatments for BPD prevention. It is also important to know that prophylactic surfactant treatment at birth of tiny infants increases the combined outcome of BPD and mortality (33), possibly due to unnecessary ventilation of some very premature infants with relatively more mature lungs. Targeted surfactant for infants with RDS is a better approach. Recently it has been possible to measure lung surfactant in gastric aspirate at birth (34,35) and clinical trials with targeted surfactant treatment based on surfactant measurements at birth compared to the standard surfactant treatment of RDS in Europe (36) are underway in Denmark. In this trial data on BPD will also be monitored.
When mechanical ventilation is needed
If necessary, nasal intermittent positive pressure ventilation (NIPPV) can often be used with success prior to IMV (37). If mechanical ventilation is required, volume targeted ventilation has been shown to decrease the combined outcome of death or BPD compared to pressure-limited ventilation (38).
Caffeine
Caffeine is recommended prophylactically in very preterm infants (36) and has been shown to reduce the risk of BPD (39) by decreasing the duration of IMV. Caffeine is a competitive adenosine receptor antagonist that stimulates the respiratory center in medulla indirectly by reducing the inhibitory effect of adenosine, and in this way decreases the frequency of apnea and the need for mechanical ventilation. In addition, caffeine increases the likelihood of successful extubation reducing the duration of mechanical ventilation.
Corticosteroids
Antenatal corticosteroid has been shown to decrease the incidence of RDS, but it has not been shown to reduce the incidence of BPD (40).
Early (<8 days of age) systemic corticosteroid treatment reduces the incidence of BPD but has more adverse effects such as hypertension, gastrointestinal bleeding and cerebral palsy (41). A systematic review and network meta-analysis evaluating fourteen different corticosteroid regimens to prevent BPD found that moderately early (8–14 days), medium cumulative dose (2–4 mg/kg), short course (<8 days), systemic dexamethasone might be the most appropriate regimen for BPD prevention and or early treatment of exudative or evolving BPD (42).
Nitric oxide (NO)
NO is produced in the nose and the concentration is around 4 parts per billion (ppb) in newborns. It influences the vascular tone in the lungs and has anti-inflammatory effects. In intubated infants auto-inhalation of endogenously produced NO is lost. In a few cases inhaled NO (iNO) has led to a reduction in death and BPD (43) and one study (44) of infants less than 34 weeks gestation who were mechanically ventilated found reduced BPD in the subgroup of infants with birth weight 1,000–1,250 g. Also, postnatal iNO in a fetal baboon model of BPD has reduced the incidence of BPD (45). However, the observed effects in randomized trials in humans have been inconsistent (46) and at present there is insufficient evidence to support the routine use of iNO in preterm babies either for prevention or treatment of BPD (47).
Oxygen saturation targeting
Though oxidant injury markers in tracheal aspirates have been linked to increased risk of BPD (48). Askie et al. in a meta-analysis (49) found no important differences in the composite outcome of death and BPD between low saturations (85–89%) vs. high saturations (91–95%). But there was a significant difference in the rates of moderate BPD (supplemental oxygen at 36 weeks PMA) favoring lower oxygen saturations (49).
Infections
Intrauterine colonization of the lungs with Ureaplasma urealyticum has been associated with increased incidence of BPD (14). Erythromycin in ventilated infants has not prevented development of BPD (50). However, other macrolides, such as azithromycin (51) and clarithromycin (52) treatment have been associated with a lower incidence of BPD. Further larger prospective studies are needed to evaluate the effect on BPD from treatment of Ureaplasma infections before this can be recommended as routine treatment.
Infections with coagulase negative staphylococci (53) and cytomegalovirus (54) have also been associated with development of BPD, but studies on prophylactic treatment and risk/ benefit analysis have not, to our knowledge been undertaken.
Optimal nutrition from birth
Decreased caloric intake and especially low protein intake decreased alveolar numbers significantly in premature rabbits (55). In preterm infants, BPD is associated with lower caloric intake and fat during the first month of life (56). Therefore, early nutritional optimization through total parenteral nutrition in addition to early enteral feeding are important in the very tiny babies to diminish BPD. Exclusive human milk-based nutrition has been shown to impact lung microbiome and decrease BPD risk. However, probiotic supplementation does not seem to affect the risk of BPD (57).
Early prediction of BPD
The literature about BPD prediction is extensive. Early prediction and intervention of BPD ideally may allow to stop or slow the progression of the disease before development of permanent sequelae.
Many authors have reported data on various biomarkers to predict BPD as early as possible before day 28. A biomarker is defined as “a characteristic that is, measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”. Biomarkers are any clinical features, radiological findings, or laboratory-based tests, that characterize disease severity, or useful to monitor disease processes and response to therapy (48). Biomarkers for prediction of BPD include genomics, microbiomics, proteomics and metabolomics (48). Several predictive risk scores for BPD have been developed based on various biomarkers, diseases and symptoms using different techniques including ‘big data’ based on artificial intelligence (AI) (31). El Faleh et al. (58) from the Swiss neonatal network primarily looked at different clinical parameters and biomarkers in 1,488 liveborn preterm infants. Using logistic regression, the authors selected the following seven variables for their predictive risk score: Antenatal steroids, median GA, median birth weight, surfactant therapy, mechanical ventilation in median days, proven infections, and patent ductus arteriosus (PDA). They evaluated their results by area under the receiver operating characteristic curves (AUCs) and found an AUC value of 0.90 for BPD day 28. Zhang et al. (59) studied 435 preterm infants from a single Chinese center. Fourteen variables that may predict BPD were analysed with Lasso regression (60) and the three most potentially useful predictors, GA, duration of mechanical ventilation, and the serum concentration of the N-terminal-pro-brain natriuretic peptide (NT-proBNP) in the first week of life were screened for the training set. The data were used to develop a nomogram to assess the risk of BPD at day 7 of life and found an AUC value of 0.85. Ding et al. (61) also from a single Chinese center studied 44 preterm infants and developed a risk score including clinical data, caloric intake and biochemical data and evaluated the score on day 40. Gursoy et al. (62) studied 652 preterm infants from a single Turkish center. They included several clinical data points including GA, birth weight, RDS, intraventricular haemorrhage, hypotension and PDA and were able to predict BPD by 72 hours after birth. Clinical complications are likely related to RDS and to the severity of RDS at this early time after birth. Concerning the scoring systems in these four studies it is obvious that the variables, namely, surfactant therapy, mechanical ventilation, infections, and PDA are not available until many days after birth and therefore are not ideal predictors. Other diagnostic tests for BPD have focused on markers linked to infections like sphingolipid metabolites (63), ceramides in tracheal aspirates (64), and changes in plasma proteome concentrations related to infections and to BPD (65). However, these metabolites need to be measured in special labs and are therefore less useful as clinical diagnostic tests. Also, neutrophil-to-lymphocyte ratio in cord blood (sensitivity 52%) and in blood at 72 hours after birth (sensitivity 61%) has been described as predictors of BPD (66). These data showed that the ratio is more reliable for neonatal infections than for prenatal infections. Larger reviews for known biomarkers of BPD are available including Cerny et al. (67) and Rivera et al. (68).
Prediction at birth
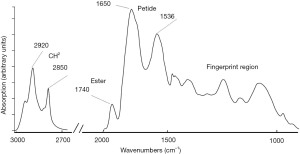
Recently it has been possible to predict BPD at birth (31) using Fourier transform mid-infrared spectroscopy (FTIR) (69) as dry transmission on gastric aspirate combined with clinical data and analysed by AI. This technology which is very rapid (results are obtained within 10–15 minutes) is reagent free and low cost. It has been pervasive in the process industry and thus well suited for deployment in acute point of care settings where speed is critical. The FTIR spectroscopy is pro tempo the only method which can produce predictive results from analyses of many molecules immediately after birth. Other methods such as mass spectroscopy are lab methods, and the results are earliest available in days (in best cases many hours) after birth. Gastric aspirate in the newborn is a fluid produced mainly in the fetal lungs (70) with contributions from the fetal kidneys and cells of the amniotic sac and therefore may include biomarkers linked to infection in cases with chorioamnionitis and correlated with intrauterine pneumonia. The sensitivity was 88% and the specificity 91% of the BPD model in a clinical study of preterm infants. An example of a FTIR spectrum of gastric aspirate from an infant born in gestational week 31 is shown in Figure 1. The clinical predictors on the day of birth were surfactant treatment, birth weight, GA, Apgar score at 5 minutes, type of delivery, need of mechanical ventilation, antenatal steroid, maternal diabetes, pre-eclampsia, intrauterine growth retardation, clinical chorioamnionitis verified by rupture of the membranes, fever, and whether there was pus in the amniotic fluid. By using Lasso regression (31,60) need for surfactant treatment was found to be the most important variable linked to BPD followed by birth weight and GA. The other clinical data did not improve the model and were not useful as predictors. The FTIR measurements of gastric aspirates were performed on a concentrate of lamellar bodies as previously described for prediction of lung maturity and RDS at birth (34). We propose that the lamellar body concentration in addition to biomarkers for lung maturity may also contain biomarkers produced in connection with feto-placental infections and other intrauterine conditions. As mentioned, the FTIR method is very fast, and the method may also be used to measure lung maturity bed-side at birth (34).
Potential early therapies and prevention in at-risk babies
More authors have published reviews of current and prospective pharmacologic therapies of BPD (5,6,67,71,72). With new methods to predict development of BPD at birth (31) the prospect of creating and testing new and more effective treatments within 24–48 hours after birth may result in prevention or less severe BPD.
Surfactant with added budesonide
Two studies have recently suggested beneficial effects of adding inhaled budesonide to surfactant for BPD prevention (73,74), and further studies are underway to confirm these findings. Focusing on selected populations at higher risk of BPD would make such studies more meaningful. Very early treatment with systemic corticosteroids has more adverse effects (41).
Surfactant with other additives
New synthetic surfactants with surfactant protein (SP) analogues of SP-B and SP-C have been shown to be clinically effective in RDS treatment. SP-D is a naturally occurring protein of the surfactant system with anti-inflammatory properties (75). Recombinant SP-D is now available and will soon be evaluated for safety in phase 1 clinical trials in preterm infants at high risk of developing BPD.
Inositol
Inositol is an important component of surfactant and trials in the 1980’s and 1990’s suggested that inositol supplementation could potentially have a role in BPD prevention (76). Recently this therapy re-emerged as a potential means of disease modification in BPD and phase-II trials have confirmed the safety and tolerability of inositol in preparation for planned efficacy studies (77).
Retinol (vitamin A), tocopherol (vitamin E), and superoxide dismutase (SOD)
Antioxidants such as retinol and tocopherol and SOD have been administered to babies in an attempt to reduce oxygen free radical induced lung damage. Of these, retinol has shown the most promise with a modest reduction in BPD found in babies treated with retinol compared with controls (78). This may be because retinol has a role in promoting alveolar septation which is reduced in “new BPD”. In animal studies topical SOD was effective at reducing lung injury, but human studies were disappointing, perhaps because of low BPD rates in the populations studied (79).
Recombinant human Clara cell 10 protein (rhCC10)
Clara cell protein developed in the respiratory epithelium was found in low concentrations in premature infants and results in reduced inflammation (80) but has not proven successful in reducing the incidence of BPD (81).
Azithromycin and macrolide therapy
Macrolide therapy has previously been used as a potential strategy for infants at risk of BPD with Ureaplasma colonisation. Azithromycin has anti-inflammatory properties in addition to antimicrobial effects and is currently being tested in a large double blind placebo controlled trial in preterm babies as a means to prevent BPD (82).
Estradiol and progesterone
Estrogen and progesterone are important in lung development and alveolar formation (83) but no significant differences between treatment groups could be identified in a randomised trial (84).
Stem cell therapy
Mesenchymal stem cell (MSC) dysfunction is thought to prevent the self-repair of immature lungs and dysfunction of MSCs increases the risk of BPD (85). Many clinical trials with MSC are currently ongoing. Very important before clinical use will be to perform safety trials and trials to determine optimal timing.
Pulmonary vasodilators, diuretics and bronchodilators
These treatments are late treatments and will not be discussed in this review.
Conclusions
BPD continues to have a high mortality and morbidity but improved understanding of the pathogenesis and the new possibility of predicting the disease at birth increases the possibility of improving the prognosis by investigation of early targeted treatment with different pharmaceuticals.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-98/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-98/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-98/coif). The study was part of a public-private partnership between the department of Pediatrics, Holbaek Hospital, Region Zealand, Denmark and SIME Diagnostics Ltd. (trading as SIME clinical AI), a private company focused on developing preventative, data-driven medicine in neonatology. HV holds part of a patent for spectroscopic analysis of biological samples. PS, NS, PV and HV hold part of a patent for prediction of bronchopulmonary dysplasia and are option holders of SIME Diagnostics Ltd. PV is CEO of SIME Diagnostics Ltd. HC is a director of Trimonocor Ltd., which is a company developing novel surfactant protein therapy. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gortner L, Misselwitz B, Milligan D, et al. Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: results from the MOSAIC cohort. Neonatology 2011;99:112-7. [Crossref] [PubMed]
- Mowitz ME, Ayyagari R, Gao W, et al. Health Care Burden of Bronchopulmonary Dysplasia Among Extremely Preterm Infants. Front Pediatr 2019;7:510. [Crossref] [PubMed]
- Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443-56. [Crossref] [PubMed]
- Thébaud B, Goss KN, Laughon M, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers 2019;5:78. [Crossref] [PubMed]
- Jain D, Bancalari E. Bronchopulmonary dysplasia: clinical perspective. Birth Defects Res A Clin Mol Teratol 2014;100:134-44. [Crossref] [PubMed]
- Capasso L, Vento G, Loddo C, et al. Oxidative Stress and Bronchopulmonary Dysplasia: Evidences From Microbiomics, Metabolomics, and Proteomics. Front Pediatr 2019;7:30. [Crossref] [PubMed]
- Elhawary NA, Tayeb MT, Abdel-Ghafar S, et al. TNF-238 polymorphism may predict bronchopulmonary dysplasia among preterm infants in the Egyptian population. Pediatr Pulmonol 2013;48:699-706. [Crossref] [PubMed]
- Watterberg KL, Demers LM, Scott SM, et al. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996;97:210-5. [Crossref] [PubMed]
- Van Marter LJ, Dammann O, Allred EN, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr 2002;140:171-6. [Crossref] [PubMed]
- Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol 2003;8:29-38. [Crossref] [PubMed]
- Payne MS, Goss KCW, Connett GJ, et al. Molecular microbiological characterization of preterm neonates at risk of bronchopulmonary dysplasia. Pediatr Res 2010;67:412-8. [Crossref] [PubMed]
- Wang EE, Ohlsson A, Kellner JD. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: results of a metaanalysis. J Pediatr 1995;127:640-4. [Crossref] [PubMed]
- Yoder BA, Coalson JJ, Winter VT, et al. Effects of antenatal colonization with ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res 2003;54:797-807. [Crossref] [PubMed]
- Schelonka RL, Katz B, Waites KB, et al. Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J 2005;24:1033-9. [Crossref] [PubMed]
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967;276:357-68. [Crossref] [PubMed]
- Groneck P, Götze-Speer B, Oppermann M, et al. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 1994;93:712-8. [Crossref] [PubMed]
- Agrons GA, Courtney SE, Stocker JT, et al. From the archives of the AFIP: Lung disease in premature neonates: radiologic-pathologic correlation. Radiographics 2005;25:1047-73. [Crossref] [PubMed]
- Hoepker A, Seear M, Petrocheilou A, et al. Wilson-Mikity syndrome: updated diagnostic criteria based on nine cases and a review of the literature. Pediatr Pulmonol 2008;43:1004-12. [Crossref] [PubMed]
- Namba F, Fujimura M, Tamura M. Bubbly and cystic appearance in chronic lung disease: Is this diagnosed as Wilson-Mikity syndrome? Pediatr Int 2016;58:251-3. [Crossref] [PubMed]
- Fujimura M, Kitajima H, Nakayama M. Increased leukocyte elastase of the tracheal aspirate at birth and neonatal pulmonary emphysema. Pediatrics 1993;92:564-9. [Crossref] [PubMed]
- Gomez Pomar E, Concina VA, Samide A, et al. Bronchopulmonary Dysplasia: Comparison Between the Two Most Used Diagnostic Criteria. Front Pediatr 2018;6:397. [Crossref] [PubMed]
- Rhodes PG, Hall RT. Continuous positive airway pressure delivered by face mask in infants with the idiopathic respiratory distress syndrome: a controlled study. Pediatrics 1973;52:1-5. [Crossref] [PubMed]
- Dunn PM. Respiratory distress syndrome. Continuous positive airway pressure (CPAP) using the Gregory box. Proc R Soc Med 1974;67:245-7. [Crossref] [PubMed]
- Avery ME, Tooley WH, Keller JB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987;79:26-30. [Crossref] [PubMed]
- Kribs A, Pillekamp F, Hünseler C, et al. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age ≤27 weeks) Paediatr Anaesth 2007;17:364-9. [Crossref] [PubMed]
- Herting E, Härtel C, Göpel W. Less invasive surfactant administration: best practices and unanswered questions. Curr Opin Pediatr 2020;32:228-34. [Crossref] [PubMed]
- Verder H, Agertoft L, Albertsen P, et al. Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study. Ugeskr Laeger 1992;154:2136-9. [PubMed]
- Verder H, Robertson B, Greisen G, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med 1994;331:1051-5. [Crossref] [PubMed]
- Verder H, Bohlin K, Kamper J, et al. Nasal CPAP and surfactant for treatment of respiratory distress syndrome and prevention of bronchopulmonary dysplasia. Acta Paediatr 2009;98:1400-8. [Crossref] [PubMed]
- Isayama T, Iwami H, McDonald S, et al. Association of Noninvasive Ventilation Strategies With Mortality and Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review and Meta-analysis. JAMA 2016;316:611-24. Erratum in: JAMA 2016;316:1116. [Crossref] [PubMed]
- Verder H, Heiring C, Ramanathan R, et al. Bronchopulmonary dysplasia predicted at birth by artificial intelligence. Acta Paediatr 2021;110:503-9. [Crossref] [PubMed]
- Villamor-Martinez E, Álvarez-Fuente M, Ghazi AMT, et al. Association of Chorioamnionitis With Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review, Meta-analysis, and Metaregression. JAMA Netw Open 2019;2:e1914611. [Crossref] [PubMed]
- Soll R. Prophylactic versus selective use of surfactant in prevention of morbidity and mortality in preterm infants. Cochrane Rev Update Neonatol 2012;102:169-71.
- Schousboe P, Verder H, Jessen TE, et al. Predicting respiratory distress syndrome at birth using fast test based on spectroscopy of gastric aspirates. 1. Biochemical part. Acta Paediatr 2020;109:280-4. [Crossref] [PubMed]
- Heiring C, Verder H, Schousboe P, et al. Predicting respiratory distress syndrome at birth using a fast test based on spectroscopy of gastric aspirates: 2. Clinical part. Acta Paediatr 2020;109:285-90. [Crossref] [PubMed]
- Sweet DG, Carnielli V, Greisen G, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2019 Update. Neonatology 2019;115:432-50. [Crossref] [PubMed]
- Shi Y, Muniraman H, Biniwale M, et al. A Review on Non-invasive Respiratory Support for Management of Respiratory Distress in Extremely Preterm Infants. Front Pediatr 2020;8:270. [Crossref] [PubMed]
- Wheeler K, Klingenberg C, McCallion N, et al. Volume-targeted versus pressure-limited ventilation in the neonate. Cochrane Database Syst Rev 2010;(11):CD003666. Update in Cochrane Database Syst Rev. 2017;10:CD003666.
- Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med 2006;354:2112-21. [Crossref] [PubMed]
- Roberts D, Brown J, Medley N, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454. [PubMed]
- Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002;109:330-8. [Crossref] [PubMed]
- Ramaswamy VV, Bandyopadhyay T, Nanda D, et al. Assessment of Postnatal Corticosteroids for the Prevention of Bronchopulmonary Dysplasia in Preterm Neonates: A Systematic Review and Network Meta-analysis. JAMA Pediatr 2021;175:e206826. [Crossref] [PubMed]
- Schreiber MD, Gin-Mestan K, Marks JD, et al. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 2003;349:2099-107. [Crossref] [PubMed]
- Kinsella JP, Cutter GR, Walsh WF, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med 2006;355:354-64. [Crossref] [PubMed]
- McCurnin DC, Pierce RA, Chang LY, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2005;288:L450-9. [Crossref] [PubMed]
- Donohue PK, Gilmore MM, Cristofalo E, et al. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics 2011;127:e414-22. [Crossref] [PubMed]
- Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev 2017;1:CD000509. [Crossref] [PubMed]
- Lal CV, Ambalavanan N. Biomarkers, Early Diagnosis, and Clinical Predictors of Bronchopulmonary Dysplasia. Clin Perinatol 2015;42:739-54. [Crossref] [PubMed]
- Askie LM, Darlow BA, Finer NAssociation Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration, et al. JAMA 2018;319:2190-201. [Crossref] [PubMed]
- Mabanta CG, Pryhuber GS, Weinberg GA, et al. Erythromycin for the prevention of chronic lung disease in intubated preterm infants at risk for, or colonized or infected with Ureaplasma urealyticum. Cochrane Database Syst Rev 2003;CD003744. [Crossref] [PubMed]
- Nair V, Loganathan P, Soraisham AS. Azithromycin and other macrolides for prevention of bronchopulmonary dysplasia: a systematic review and meta-analysis. Neonatology 2014;106:337-47. [Crossref] [PubMed]
- Ozdemir R, Erdeve O, Dizdar EA, et al. Clarithromycin in preventing bronchopulmonary dysplasia in Ureaplasma urealyticum-positive preterm infants. Pediatrics 2011;128:e1496-501. [Crossref] [PubMed]
- Liljedahl M, Bodin L, Schollin J. Coagulase-negative staphylococcal sepsis as a predictor of bronchopulmonary dysplasia. Acta Paediatr 2004;93:211-5. [Crossref] [PubMed]
- Sawyer MH, Edwards DK, Spector SA. Cytomegalovirus infection and bronchopulmonary dysplasia in premature infants. Am J Dis Child 1987;141:303-5. [PubMed]
- Mataloun MM, Rebello CM, Mascaretti RS, et al. Pulmonary responses to nutritional restriction and hyperoxia in premature rabbits. J Pediatr (Rio J) 2006;82:179-85. [Crossref] [PubMed]
- Akram Khan M, Kuzma-O'Reilly B, Brodsky NL, et al. Site-specific characteristics of infants developing bronchopulmonary dysplasia. J Perinatol 2006;26:428-35. [Crossref] [PubMed]
- Villamor-Martínez E, Pierro M, Cavallaro G, et al. Probiotic Supplementation in Preterm Infants Does Not Affect the Risk of Bronchopulmonary Dysplasia: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2017;9:1197. [Crossref] [PubMed]
- El Faleh I, Faouzi M, Adams M, et al. Bronchopulmonary dysplasia: a predictive scoring system for very low birth weight infants. A diagnostic accuracy study with prospective data collection. Eur J Pediatr 2021;180:2453-61. [Crossref] [PubMed]
- Zhang J, Luo C, Lei M, et al. Development and Validation of a Nomogram for Predicting Bronchopulmonary Dysplasia in Very-Low-Birth-Weight Infants. Front Pediatr 2021;9:648828. [Crossref] [PubMed]
- Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol 1996;58:267-88.
- Ding L, Wang H, Geng H, et al. Prediction of Bronchopulmonary Dysplasia in Preterm Infants Using Postnatal Risk Factors. Front Pediatr 2020;8:349. [Crossref] [PubMed]
- Gursoy T, Hayran M, Derin H, et al. A clinical scoring system to predict the development of bronchopulmonary dysplasia. Am J Perinatol 2015;32:659-66. [PubMed]
- Hendricks-Muñoz KD, Xu J, Voynow JA. Tracheal aspirate VEGF and sphingolipid metabolites in the preterm infant with later development of bronchopulmonary dysplasia. Pediatr Pulmonol 2018;53:1046-52. [Crossref] [PubMed]
- van Mastrigt E, Zweekhorst S, Bol B, et al. Ceramides in tracheal aspirates of preterm infants: Marker for bronchopulmonary dysplasia. PLoS One 2018;13:e0185969. [Crossref] [PubMed]
- Zasada M, Suski M, Bokiniec R, et al. Comparative two time-point proteome analysis of the plasma from preterm infants with and without bronchopulmonary dysplasia. Ital J Pediatr 2019;45:112. [Crossref] [PubMed]
- Sun Y, Chen C, Zhang X, et al. High Neutrophil-to-Lymphocyte Ratio Is an Early Predictor of Bronchopulmonary Dysplasia. Front Pediatr 2019;7:464. [Crossref] [PubMed]
- Cerny L, Torday JS, Rehan VK. Prevention and treatment of bronchopulmonary dysplasia: contemporary status and future outlook. Lung 2008;186:75-89. [Crossref] [PubMed]
- Rivera L, Siddaiah R, Oji-Mmuo C, et al. Biomarkers for Bronchopulmonary Dysplasia in the Preterm Infant. Front Pediatr 2016;4:33. [Crossref] [PubMed]
- De Bruyne S, Speeckaert MM, Delanghe JR. Applications of mid-infrared spectroscopy in the clinical laboratory setting. Crit Rev Clin Lab Sci 2018;55:1-20. [Crossref] [PubMed]
- Spillman T, Cotton DB. Current perspectives in assessment of fetal pulmonary surfactant status with amniotic fluid. Crit Rev Clin Lab Sci 1989;27:341-89. [Crossref] [PubMed]
- Roberts K, Stepanovich G, Bhatt-Mehta V, et al. New Pharmacologic Approaches to Bronchopulmonary Dysplasia. J Exp Pharmacol 2021;13:377-96. [Crossref] [PubMed]
- Michael Z, Spyropoulos F, Ghanta S, et al. Bronchopulmonary Dysplasia: An Update of Current Pharmacologic Therapies and New Approaches. Clin Med Insights Pediatr 2018;12:1179556518817322. [Crossref] [PubMed]
- Kothe TB, Sadiq FH, Burleyson N, et al. Surfactant and budesonide for respiratory distress syndrome: an observational study. Pediatr Res 2020;87:940-5. [Crossref] [PubMed]
- Yeh TF, Chen CM, Wu SY, et al. Intratracheal Administration of Budesonide/Surfactant to Prevent Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 2016;193:86-95. [Crossref] [PubMed]
- Watson A, Madsen J, Clark HW SP-A. Front Immunol 2020;11:622598. [Crossref] [PubMed]
- Hallman M, Järvenpää AL, Pohjavuori M. Respiratory distress syndrome and inositol supplementation in preterm infants. Arch Dis Child 1986;61:1076-83. [Crossref] [PubMed]
- Phelps DL, Ward RM, Williams RL, et al. Safety and pharmacokinetics of multiple dose myo-inositol in preterm infants. Pediatr Res 2016;80:209-17. [Crossref] [PubMed]
- Araki S, Kato S, Namba F, et al. Vitamin A to prevent bronchopulmonary dysplasia in extremely low birth weight infants: a systematic review and meta-analysis. PLoS One 2018;13:e0207730. [Crossref] [PubMed]
- Suresh GK, Davis JM, Soll RF. Superoxide dismutase for preventing chronic lung disease in mechanically ventilated preterm infants. Cochrane Database Syst Rev 2001;CD001968. [Crossref] [PubMed]
- Levine CR, Gewolb IH, Allen K, et al. The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatr Res 2005;58:15-21. [Crossref] [PubMed]
- Davis JM, Pilon AL, Shenberger J, et al. The role of recombinant human CC10 in the prevention of chronic pulmonary insufficiency of prematurity. Pediatr Res 2019;86:254-60. [Crossref] [PubMed]
- Lowe J, Gillespie D, Hubbard M, et al. Study protocol: azithromycin therapy for chronic lung disease of prematurity (AZTEC) - a randomised, placebo-controlled trial of azithromycin for the prevention of chronic lung disease of prematurity in preterm infants. BMJ Open 2020;10:e041528. [Crossref] [PubMed]
- Trotter A, Ebsen M, Kiossis E, et al. Prenatal estrogen and progesterone deprivation impairs alveolar formation and fluid clearance in newborn piglets. Pediatr Res 2006;60:60-4. [Crossref] [PubMed]
- Trotter A, Maier L, Kron M, et al. Effect of oestradiol and progesterone replacement on bronchopulmonary dysplasia in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2007;92:F94-8. [Crossref] [PubMed]
- Mandell EW, Kratimenos P, Abman SH, et al. Drugs for the Prevention and Treatment of Bronchopulmonary Dysplasia. Clin Perinatol 2019;46:291-310. [Crossref] [PubMed]
Cite this article as: Verder H, Li ZK, Ramanathan R, Clark H, Sweet DG, Schousboe P, Scoutaris N, Verder P, Heiring C. Bronchopulmonary dysplasia with focus on early prediction and treatment: a narrative review. Pediatr Med 2023;6:13.

