Respiratory system abnormalities in Prader-Willi syndrome: a literature review
Introduction
Prader-Willi syndrome (PWS) is a genetic condition resulting from errors in genomic imprinting of the chromosome 15q11-q13 region, most frequently due to a de novo paternal deletion of the chromosome (about 60% of cases) leading to a lack of expression of paternally derived genes (1). Maternal uniparental disomy or both chromosome 15s inherited from the mother is seen in about 35% of cases (2,3). Micro-deletions and epimutations in the genomic imprinting center occur in about 3% of cases (2). Rarely, chromosomal translocations or rearrangements of the 15q11-q13 region are reported (3).
PWS affects multiple organ systems and presents with certain clinical features in different growth stages as patients go through a series of nutritional phases (4-7). Significant hypotonia, poor feeding, and failure to thrive occur in infancy. After this period, PWS patients start gaining weight, and between the ages of 3–15 years (with median age of 8 years), there is onset of very aggressive food seeking behavior, reduced satiety and hyperphagia resulting in obesity (7). Obesity is a hallmark feature of PWS, and growth hormone deficiency (GHD) is common with a prevalence ranging from 40–100% based on various studies (8-10). Hypogonadism is also a prominent feature, manifesting as cryptorchidism and micropenis in males (11) and hypoplasia of the external genitalia in females (12). Learning disabilities and mild to moderate intellectual impairment, as well as behavioral issues like skin picking and temper tantrums are common (13).
Despite the vast progress over the past decades in the diagnosis and management, the annual mortality rate of individuals with PWS is still higher than for the general population (14-16). Whittington et al. estimated a death rate of 3% per annum in PWS patients compared to that of 1% per annum in the general population (15). One of the largest published cohorts of French PWS registry reported 104 deaths over 11 years. The median age of death was 30 years, ranging from 0–58 years. Respiratory causes accounted for >50% of the mortality, and while respiratory failure was responsible for deaths in adults, respiratory infections were the primary cause of death in children (17). In a series of 64 PWS patients up to 19 years of age, 61% of deaths were due to a respiratory disorder with 44%of these following an upper respiratory tract infection and the remainder due to suffocation or sudden death during sleep, independent of growth hormone treatment (GHT). The median age at death was 3 years (18). The USA PWS Association examined the survival trends in PWS and showed that respiratory failure was the leading cause of death in both males and females, accounting for one-third of all deaths (19).
The pathogenesis of the respiratory problems in PWS seems to be multifactorial in origin, including abnormalities in the control of breathing, swallowing dysfunction and aspiration, respiratory system infections, sleep disordered breathing (SDB), and respiratory failure. Patients with PWS thus need careful otolaryngology, respiratory and dietary evaluation as part of their ongoing management and follow-up.
This review summarizes and updates the current knowledge about the mechanisms and screening recommendations for respiratory system abnormalities associated with PWS. This is pertinent as respiratory infections and respiratory failure are the leading causes of mortality in individuals with PWS, and clinicians need to be aware of risk stratification of patients with respiratory issues prior to initiation of growth hormone (GH) therapy, due to reports of unexpected mortality with GH therapy in a subset of these patients. We present this article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-102/rc).
Methods
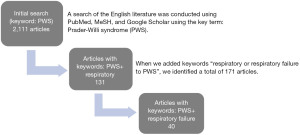
Literature search was conducted via PubMed, MeSH, and Google Scholar with keywords that included “Prader-Willi syndrome” and “respiratory” (Figure 1). Additional papers were found via references from articles related to the original search. The articles written in English were selected for review (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | Multiple dates |
| Databases and other sources searched | PubMed, MeSH, and Google Scholar |
| Search terms used | PWS |
| PWS and respiratory failure | |
| PWS and GHT | |
| Central and OSA | |
| Time frame | January 1, 1976, and December 31, 2021 |
| Inclusion and exclusion criteria | Clinical trial |
| Meta-analysis | |
| Randomized controlled trial | |
| Review | |
| Systematic review | |
| Language: English | |
| Selection process | All authors equally conducted the selection, reviewed and agreed with the selected article. |
PWS, Prader-Willi Syndrome; GHT, growth hormone treatment; OSA, obstructive sleep apnea.
Discussion
Respiratory control abnormalities
Regulation of the control of breathing is governed by the central and peripheral chemoreceptors. While central chemoreceptors react to changes in carbon dioxide tension (PCO2) and hydrogen (H+) concentration, peripheral chemoreceptors respond to the changes in (PCO2) and low oxygen partial pressure (PaO2) levels (20). In mammals, response to hypercapnia and hypoxia is accomplished by increasing the minute ventilation that in turn maintains blood oxygen levels in the normal range. Studies have shown peripheral chemoreceptor function abnormalities in both obese and nonobese patients with PWS (21,22).
Hypoxic ventilatory response (HPVR)
Abnormalities in HPVR have been shown in individuals with PWS. Gozal et al. showed that the initial hyperoxic challenge resulted in decrease in minute ventilation in the control subjects but a paradoxical response of increased minute ventilation was observed in subjects with PWS (21). Transient and isocapnic hypoxia challenge during wakefulness resulted in a marked increase in minute ventilation in healthy controls. However, patients with PWS demonstrated absent or reduced ventilatory responses to the same challenge. These observations imply that peripheral chemoreceptor function is affected in both obese and nonobese patients with PWS (21). Another study showed that individuals with PWS demonstrated abnormal arousal and cardiorespiratory responses to hypoxia (22).
Hypercapnic respiratory response (HCVR)
Hypercapnia is the principal stimulus for the respiratory drive in normal physiological states (23). It has been shown that obese subjects with PWS had a blunted HCVR as opposed to non-obese subjects with PWS and body mass index (BMI)-matched obese controls. This blunted ventilatory response to hypercarbic challenge was attributed to peripheral chemoreceptor dysfunction and/or defect in the afferent pathways projecting to central controllers of breathing (21,22). All these abnormalities in HPVR, HCVR, and arousal response observed in PWS may contribute to morbidity and mortality in these patients. GHT has been shown to improve ventilatory response to hypercarbia and central inspiratory drive in children with PWS (24,25).
Dysphagia/silent aspiration
Infantile hypotonia is a very characteristic feature of PWS and is associated with feeding difficulties during the newborn period (4,7). Most infants with PWS have a weak or uncoordinated swallow that puts them at risk for aspiration (4,26). In general, children with hypotonia may not exhibit the overt, typical symptoms of aspiration such as coughing, gagging, and choking. Subtle signs of “silent” aspiration can be missed, and children may present with apnea, wheezing, or wet cough (27). Recent reports indicate that persons with PWS likely to have an undetected swallowing problem even during adulthood (27,28). A study performed in 30 individuals with PWS between ages of 5–35 years showed that although none of the participants displayed overt signs of dysphagia, majority had abnormal video fluoroscopy swallow study (VFSS) indicating that the dysphagia is subclinical and cannot be detected without formal testing (27). Another retrospective study of 10 infants and toddlers with PWS (age 3 weeks to 29 months) showed a high rate of swallowing dysfunction in the subjects (28). All aspiration events were silent and there were no differences in the rates of aspiration for gender, genetic subtype, or GHT (28). These studies indicate that persons with PWS may present with undetected swallowing dysfunction, even beyond infancy (3). Today, early diagnosis, multidisciplinary care, and GHT have decreased hypotonia, the duration of tube feeding, and dysphagia in children with PWS (3,29,30).
Chest wall and pulmonary mechanics
Hypotonia and respiratory muscle weakness
Hypotonia in PWS presents in utero and is associated with decreased fetal movements and atypical fetal positioning. In the majority of cases, affected newborns are severely hypotonic with depressed reflex activity (4,6). In rare cases, children may present with severe respiratory muscle weakness similar to children with neuromuscular disease complicated with abnormal airway clearance, secretion retention and lower airway infections (26). Later in life, while the hypotonia associated with respiratory muscle weakness alone is usually not severe enough to result in respiratory compromise, when it is coupled with scoliosis and if the ventilatory load is increased secondary to obesity, a restrictive ventilatory defect may present resulting in nocturnal hypoventilation (26,31,32). Although underlying pathogenesis of muscle weakness, and other motor problems in PWS patients is not clear, it is likely that abnormal body composition with an increase in fat mass, decrease in lean body mass (LBM), and some degree of other neuromuscular abnormalities are contributing factors (31,33,34).
Obesity
To maintain the target minute ventilation and compensate the low tidal volume breathing, obese individuals present with a rapid and shallow breathing pattern. They also demonstrate a decreased exercise capacity, a heightened demand for ventilation, elevated work of breathing, and diminished respiratory compliance (35). In obese individuals, conventional respiratory function tests show only mild abnormalities except in extreme cases. The mass loading effect of obesity decreases functional reserve capacity (FRC) while the residual volume (RV) remains normal, leading to decline in expiratory reserve volume (ERV). In obese subjects, accumulation of the fat tissue in the abdomen, around the diaphragm, and ribs decreases the compliance of the respiratory system to the one-third of the normal level (36). In obese children with PWS, alterations in pulmonary function may be more greatly affected due to abnormal body compositions and respiratory muscle weakness which are more prominent in GH naive children (25,26). Early diagnosis, optimal treatment with multidisciplinary care have improved outcomes of individuals with PWS (37). GHT is the major proven intervention for the individuals with PWS and was approved by the US Food and Drug Administration in 2000. Since then, several benefits of GHT have been shown including increasing height, decreasing body fat, increasing muscle mass, improving weight distribution, increasing physical activity and exercise capacity, and improving bone health (8,38). In addition, GHT has been shown to have a positive effect on development, behavior, cognition and quality of life in PWS (8,30,39).
Sleep and breathing
Individuals with PWS frequently present with a variety of sleep problems, such as central sleep apnea (CSA), obstructive sleep apnea (OSA), alveolar hypoventilation, altered sleep architecture, excessive daytime sleepiness, and narcolepsy-like symptoms (25,26). Individuals with PWS have a high prevalence of sleep related hypoxemia and hypoventilation as well as high respiratory disturbance index (RDI), especially during rapid eye movement (REM) sleep. Comparing to the control subjects with a similar level of obesity, persons with PWS spend more time with low oxygen saturation (SpO2) levels due to SDB (40). The prevalence of sleep related respiratory events in PWS varies significantly, ranging 41% to 80% (41). There is also an age-related difference in the patterns of SDB in children with PWS. A study performed in GHT naïve infants and children with PWS who did not necessarily have symptoms suggestive of SDB showed that CSA (defined as a central apnea index >5/hour) was common in less than 1 year of age (42). The study also showed that while CSA was uncommon beyond 2 years of age, obstructive events were more common in older children (42). Similarly, another report from China involving 48 children with PWS showed that infants were more likely to have CSA (71.8%) as opposed to older children (25%) who were more likely to have OSA (43).
The Clinical Advisory Board of the PWS Association recommends an overnight polysomnography performed in all children with PWS (5). Today, younger age at diagnosis, multidisciplinary management, and early interventions including GHT have been changing the natural history of PWS and related morbidities including SDB.
CSA
CSA are well recognized in PWS, seems to be more common in children <2 years of age, occur more frequently in REM sleep, and can result in hypoxemia (26,41). The etiology of CSA is likely multifactorial that include poor muscle tone, brainstem immaturity, hypothalamic dysfunction, and altered chemosensitivity to carbon dioxide (CO2) (25,44). It has been proposed that blunted CO2 responses in PWS may results in overshooting of HCVR that in turn results in a fall in partial pressure of carbon dioxide (PaCO2) below the eucapnic level and apneic threshold (26). CSA may cause desaturation or be terminated by an arousal and both stimuli may further perpetuate this cycle. It has been shown that oxygen administration significantly improved CSA in children with PWS by eliminating the hypoxia as a precipitating factor and by stabilizing the breathing pattern (45). CSA reported to be more common in PWS individuals with central adrenal insufficiency (44). CSA improves in the majority of infants (73%) beyond 2 years of life, likely due to the maturation of the brainstem by time (41,42).
Necdin, one of a cluster of genes deleted in PWS, may account for respiratory, sensory, motor and behavioral problems associated with PWS. Zanella et al. reported that Necdin-deficiency in mice induces central respiratory deficits similar to PWS such as irregular rhythm, frequent apneas, and blunted respiratory regulations (46). Necdin is expressed by medullary serotonergic neurons, and necdin deficiency in neonatal mice alters the serotonergic modulation of the respiratory rhythm generator (47). Studies using genetically altered mice showed that necdin deficiency resulted in serotonergic neuro-structural changes and increased expression and activity of serotonin transporters (47). All these findings support the hypothesis that brainstem serotonopathy is likely part of the pathophysiology of the respiratory and behavioral symptoms of PWS (47).
Although the prevalence of CSA decreases in older subjects with PWS, abnormalities of respiratory control during sleep including blunted ventilatory response to hypercapnia and hypoxia, and poor arousal and cardiorespiratory responses to hypoxia may persist. These deficits may predispose individuals with PWS to developing sleep-related hypoventilation later in life (25). In addition, the abnormal responses to hypoxia and hypercapnia can be exacerbated by obesity. In severe cases, obesity hypoventilation syndrome can develop with significant nocturnal and daytime hypercapnia and these patients may present with symptoms such as hypersomnolence, fatigue or morning headaches and chronic nocturnal hypoxemia can lead to polycythemia and pulmonary hypertension (26). However, today, many people with PWS are not very obese and these extreme conditions are rarely seen in patients who are receiving guideline based optimal treatments.
OSA
The prevalence of OSA in children with PWS ranges from 44% to 100%, while the rate is 2–3% among healthy children (41,48,49). Individuals with PWS have multiple risk factors for OSA such as craniofacial abnormalities associated with small upper airway caliber, airway obstruction caused by obesity related fat deposition, pharyngeal muscular hypotonia leading to airway collapsibility, and hypertrophic tonsils and adenoids narrowing the airway (42,48). Imaging studies of persons with PWS have shown reduced cross-sectional area at the oropharyngeal or nasopharyngeal level, and although most infants with PWS are not obese, increased airway fat deposition has been demonstrated even in this stage of growth (50). Studies also have shown a varying frequency of central hypothyroidism in PWS from 2–4% to as high as 20–30%, that may be a contributing factor for the sleep related breathing abnormalities (51,52).
In a retrospective report, Pavone et al. studied 88 PWS patients (median age 5.1 years) from 3 centers. The prevalence of SDB, defined as apnea hypopnea index (AHI) ≥1.5/h in children and ≥5/h in adults, was 53% in children and 41% in adult subjects. The respiratory events were predominantly obstructive in nature (53). In 30 PWS children from Taiwan (mean age 7.4±4.1 years), the prevalence of SDB, defined by a RDI of >2/hour, was 93%. While 47.4% of the apneas were central, 52.6% were obstructive (54). Sedky et al. analyzed fourteen studies of children with PWS who were assessed with polysomnography (n=224). The prevalence of OSA across studies was 79.91%. Among subjects with OSA, 53.07% had mild OSA, 22.35% moderate OSA, and 24.58% severe OSA (48). A prospective study from China enrolled 48 children with PWS. The median age of the children was 16.8 months, ranging between 3–188 months. Overnight polysomnography of these children showed that 87.5% of them had SDB. While 11 (20%) had OSA, 27 (56%) patients had CSA, and 4 patients had both OSA and CSA indicating that while younger children mostly presents with CSA, the prevalence of OSA increases by age (43).
Adenotonsillectomy (AT) is the treatment of choice in children with OSA including those with PWS (49,55). Sedky et al. showed that although AT was associated with improvement in OSA for most children with PWS, residual OSA was present in the majority of cases following surgery (48). Two recent metanalysis investigated the outcomes of AT for OSA in children with PWS showed that 20% of patients had complete resolution of OSA (AHI <1.5) and 67% had improvement in the severity of OSA (49,56). They also have found that post op complications of AT were more common (such as velopharyngeal insufficiency) in PWS than non-syndromic children with OSA. These results indicate that although complete resolution of OSA in PWS is not common, many patients benefit from this surgery. Therefore, any patient with PWS and symptoms suggestive of OSA should be evaluated by a polysomnography and referred to otolaryngologist for the surgical management.
SDB and GHT
Early reports of sudden death within first 9 months of GHT have raised concerns about this treatment in PWS (57-60). Initially, it was thought that GHT would worsen the severity of OSA by increasing the soft tissue mass of the airway. However, retrospective reviews of sudden death incidents in individuals with PWS showed that there was no difference in the rates of death who were on or off of GHT (61-63).
GHT improves bone mineral density, body composition, muscle strength, height, and BMI in children with PWS (64). In addition, GHT seems to improve cognitive function and long-term health-related quality of life (39). The FDA recommends GHT at 2 years of age, however, many experts prefers to start GH therapy as early as 2–3 months of age due to its beneficial effects on motor and neurocognitive development (8,39,65).
Several studies investigated the impact of GHT on polysomnography findings of SDB in PWS (66,67). One study involved 62 children aged between 0 and 2.5 years at the beginning of the study. Twenty-one subjects started GHT during the first year of life and 41 after the first year of life (68). Polysomnographic data acquired before and after GHT in regular intervals. There were no significant differences in the RDIs including obstructive apnea hypopnea, central apnea, oxygen desaturation index, and average SpO2 levels between children who were treated with GH during the first year of life versus the ones received treatment after 1 year of age (68). In an Australian multicenter retrospective analysis, children with PWS investigated with polysomnography before and after introduction of GHT. The study has shown that the median obstructive AHI for the group did not increase significantly after the initiation of GHT. However, 13% of children with no or mild OSA at baseline developed moderate/severe OSA after the initiation of GH therapy (69). Development of OSA could not be explained by changes in body weight and appeared to be more pronounced in the children under 3 years of age. It is likely that adenoid ± tonsillar tissue hypertrophy which is a common finding in this age group, may have caused OSA in this small percentage of study subjects (70). In this cohort, there was no evidence of a change in CSA with GHT (69).
GHT is currently approved for adults with PWS in a few countries. While the prevalence of OSA in children with PWS has been reported to be as high as 80% (49), the prevalence of moderate to severe OSA has been reported around 22% in adults with PWS (71). A longitudinal prospective study that investigated the effects of GHT on sleep parameters in adults with PWS included thirty-seven adults who were randomly assigned to 1 year of GHT (n=19) or placebo (n=18) followed by 2 years of GHT to all. Each subject underwent polysomnography every 6 months. The study showed a baseline AHI 1.4 (range, 0.0–13.9). There were no differences in sleep or respiratory parameters between GH and placebo-treated patients (72).
A recent report reviewed the published studies of GHT in adults with PWS showed that in most reports the treatment duration was only 1–2 years, except two studies followed patients up to 5 years (73). Although GHT has been shown to help maintain normal body composition and metabolism as well as increased the quality of life, long-term benefits or possible adverse effects of GHT on the respiratory system in adults with PWS need to be investigated further.
Conclusions
Despite the vast progress over the past decades in the diagnosis and management, the annual mortality rate of individuals with PWS is still higher than the general population. Therefore, early diagnosis and multidisciplinary management are crucial for the survival of individuals with PWS. Multiple factors contribute to the respiratory problems seen in PWS, including abnormalities in the control of breathing, swallowing dysfunction and aspiration, respiratory system infections, SDB and respiratory failure. Best practice guidelines recommend a multidisciplinary approach to the diagnosis and management of patients with PWS, including a sleep medicine specialist with expertise of PWS (5,8). GHT is safe and well-tolerated in pediatric population with PWS. The guidelines recommend a polysomnography screening prior to initiating of the GHT, 3–6 months after starting treatment, and then annual screening (8,74). Holding GHT is recommended if there is a concern for development of any type of SDB until it is cleared by an overnight polysomnography (74).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine for the series “Clinical Pearls in Pediatric Endocrinology and Metabolism”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-102/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-102/coif). The series “Clinical Pearls in Pediatric Endocrinology and Metabolism” was commissioned by the editorial office without any funding or sponsorship. BEL served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Pediatric Medicine from October 2022 to September 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nicholls RD, Knoll JH, Butler MG, et al. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature 1989;342:281-5. [Crossref] [PubMed]
- Butler MG, Hartin SN, Hossain WA, et al. Molecular genetic classification in Prader-Willi syndrome: a multisite cohort study. J Med Genet 2019;56:149-53. [Crossref] [PubMed]
- Butler MG, Miller JL, Forster JL. Prader-Willi Syndrome - Clinical Genetics, Diagnosis and Treatment Approaches: An Update. Curr Pediatr Rev 2019;15:207-44. [Crossref] [PubMed]
- Wang P, Zhou W, Yuan W, et al. Prader-Willi syndrome in neonates: twenty cases and review of the literature in Southern China. BMC Pediatr 2016;16:124. [Crossref] [PubMed]
- Duis J, van Wattum PJ, Scheimann A, et al. A multidisciplinary approach to the clinical management of Prader-Willi syndrome. Mol Genet Genomic Med 2019;7:e514. [Crossref] [PubMed]
- Cassidy SB, Schwartz S, Miller JL, et al. Prader-Willi syndrome. Genet Med 2012;14:10-26. [Crossref] [PubMed]
- Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A 2011;155A:1040-9. [Crossref] [PubMed]
- Deal CL, Tony M, Höybye C, et al. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab 2013;98:E1072-87. [Crossref] [PubMed]
- Burman P, Ritzén EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev 2001;22:787-99. [Crossref] [PubMed]
- Diene G, Mimoun E, Feigerlova E, et al. Endocrine disorders in children with Prader-Willi syndrome--data from 142 children of the French database. Horm Res Paediatr 2010;74:121-8. [Crossref] [PubMed]
- Eiholzer U, l'Allemand D, Rousson V, et al. Hypothalamic and gonadal components of hypogonadism in boys with Prader-Labhart- Willi syndrome. J Clin Endocrinol Metab 2006;91:892-8. [Crossref] [PubMed]
- Crinò A, Schiaffini R, Ciampalini P, et al. Hypogonadism and pubertal development in Prader-Willi syndrome. Eur J Pediatr 2003;162:327-33. [Crossref] [PubMed]
- Roof E, Stone W, MacLean W, et al. Intellectual characteristics of Prader-Willi syndrome: comparison of genetic subtypes. J Intellect Disabil Res 2000;44:25-30. [Crossref] [PubMed]
- Einfeld SL, Kavanagh SJ, Smith A, et al. Mortality in Prader-Willi syndrome. Am J Ment Retard 2006;111:193-8. [Crossref] [PubMed]
- Whittington JE, Holland AJ, Webb T, et al. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet 2001;38:792-8. [Crossref] [PubMed]
- Proffitt J, Osann K, McManus B, et al. Contributing factors of mortality in Prader-Willi syndrome. Am J Med Genet A 2019;179:196-205. [Crossref] [PubMed]
- Pacoricona Alfaro DL, Lemoine P, Ehlinger V, et al. Causes of death in Prader-Willi syndrome: lessons from 11 years' experience of a national reference center. Orphanet J Rare Dis 2019;14:238. [Crossref] [PubMed]
- Tauber M, Diene G, Molinas C, et al. Review of 64 cases of death in children with Prader-Willi syndrome (PWS). Am J Med Genet A 2008;146A:881-7. [Crossref] [PubMed]
- Butler MG, Manzardo AM, Heinemann J, et al. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med 2017;19:635-42. [Crossref] [PubMed]
- Pamenter ME, Powell FL. Time Domains of the Hypoxic Ventilatory Response and Their Molecular Basis. Compr Physiol 2016;6:1345-85. [Crossref] [PubMed]
- Gozal D, Arens R, Omlin KJ, et al. Absent peripheral chemosensitivity in Prader-Willi syndrome. J Appl Physiol (1985) 1994;77:2231-6. [Crossref] [PubMed]
- Arens R, Gozal D, Burrell BC, et al. Arousal and cardiorespiratory responses to hypoxia in Prader-Willi syndrome. Am J Respir Crit Care Med 1996;153:283-7. [Crossref] [PubMed]
- Goldberg S, Ollila HM, Lin L, et al. Analysis of Hypoxic and Hypercapnic Ventilatory Response in Healthy Volunteers. PLoS One 2017;12:e0168930. [Crossref] [PubMed]
- Lindgren AC, Hellström LG, Ritzén EM, et al. Growth hormone treatment increases CO(2) response, ventilation and central inspiratory drive in children with Prader-Willi syndrome. Eur J Pediatr 1999;158:936-40. [Crossref] [PubMed]
- Gillett ES, Perez IA. Disorders of Sleep and Ventilatory Control in Prader-Willi Syndrome. Diseases 2016;4:23. [Crossref] [PubMed]
- Tan HL, Urquhart DS. Respiratory Complications in Children with Prader Willi Syndrome. Paediatr Respir Rev 2017;22:52-9. [Crossref] [PubMed]
- Gross RD, Gisser R, Cherpes G, et al. Subclinical dysphagia in persons with Prader-Willi syndrome. Am J Med Genet A 2017;173:384-94. [Crossref] [PubMed]
- Salehi P, Stafford HJ, Glass RP, et al. Silent aspiration in infants with Prader-Willi syndrome identified by videofluoroscopic swallow study. Medicine (Baltimore) 2017;96:e9256. [Crossref] [PubMed]
- Bacheré N, Diene G, Delagnes V, et al. Early diagnosis and multidisciplinary care reduce the hospitalization time and duration of tube feeding and prevent early obesity in PWS infants. Horm Res 2008;69:45-52. [PubMed]
- Passone CGB, Franco RR, Ito SS, et al. Growth hormone treatment in Prader-Willi syndrome patients: systematic review and meta-analysis. BMJ Paediatr Open 2020;4:e000630. [Crossref] [PubMed]
- Reus L, Zwarts M, van Vlimmeren LA, et al. Motor problems in Prader-Willi syndrome: a systematic review on body composition and neuromuscular functioning. Neurosci Biobehav Rev 2011;35:956-69. [Crossref] [PubMed]
- Hákonarson H, Moskovitz J, Daigle KL, et al. Pulmonary function abnormalities in Prader-Willi syndrome. J Pediatr 1995;126:565-70. [Crossref] [PubMed]
- Butte NF, Hopkinson JM, Wong WW, et al. Body composition during the first 2 years of life: an updated reference. Pediatr Res 2000;47:578-85. [Crossref] [PubMed]
- Forbes GB. A distinctive obesity: body composition provides the clue. Am J Clin Nutr 1997;65:1540-1. [Crossref] [PubMed]
- Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J 2006;13:203-10. [Crossref] [PubMed]
- Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med 2018;12:755-67. [Crossref] [PubMed]
- Eiholzer U. Deaths in children with Prader-Willi syndrome. A contribution to the debate about the safety of growth hormone treatment in children with PWS. Horm Res 2005;63:33-9. [PubMed]
- Lindgren AC, Lindberg A. Growth hormone treatment completely normalizes adult height and improves body composition in Prader-Willi syndrome: experience from KIGS (Pfizer International Growth Database). Horm Res 2008;70:182-7. [PubMed]
- Deal CL, Rogol AD. Growth hormone treatments and cognitive functioning in children with Prader-Willi syndrome. Eur J Endocrinol 2020;182:C21-5. [Crossref] [PubMed]
- Miller J, Wagner M. Prader-Willi syndrome and sleep-disordered breathing. Pediatr Ann 2013;42:200-4. [Crossref] [PubMed]
- Cataldi M, Arnaldi D, Tucci V, et al. Sleep disorders in Prader-Willi syndrome, evidence from animal models and humans. Sleep Med Rev 2021;57:101432. [Crossref] [PubMed]
- Cohen M, Hamilton J, Narang I. Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi Syndrome. PLoS One 2014;9:e101012. [Crossref] [PubMed]
- Lu A, Luo F, Sun C, et al. Sleep-disordered breathing and genetic findings in children with Prader-Willi syndrome in China. Ann Transl Med 2020;8:989. [Crossref] [PubMed]
- de Lind van Wijngaarden RF, Joosten KF, van den Berg S, et al. The relationship between central adrenal insufficiency and sleep-related breathing disorders in children with Prader-Willi syndrome. J Clin Endocrinol Metab 2009;94:2387-93. [Crossref] [PubMed]
- Urquhart DS, Gulliver T, Williams G, et al. Central sleep-disordered breathing and the effects of oxygen therapy in infants with Prader-Willi syndrome. Arch Dis Child 2013;98:592-5. [Crossref] [PubMed]
- Zanella S, Watrin F, Mebarek S, et al. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J Neurosci 2008;28:1745-55. [Crossref] [PubMed]
- Wu RN, Hung WC, Chen CT, et al. Firing activity of locus coeruleus noradrenergic neurons decreases in necdin-deficient mice, an animal model of Prader-Willi syndrome. J Neurodev Disord 2020;12:21. [Crossref] [PubMed]
- Sedky K, Bennett DS, Pumariega A. Prader Willi syndrome and obstructive sleep apnea: co-occurrence in the pediatric population. J Clin Sleep Med 2014;10:403-9. [Crossref] [PubMed]
- Clements AC, Dai X, Walsh JM, et al. Outcomes of Adenotonsillectomy for Obstructive Sleep Apnea in Prader-Willi Syndrome: Systematic Review and Meta-analysis. Laryngoscope 2021;131:898-906. [Crossref] [PubMed]
- Richards A, Quaghebeur G, Clift S, et al. The upper airway and sleep apnoea in the Prader-Willi syndrome. Clin Otolaryngol Allied Sci 1994;19:193-7. [Crossref] [PubMed]
- Butler MG, Theodoro M, Skouse JD. Thyroid function studies in Prader-Willi syndrome. Am J Med Genet A 2007;143A:488-92. [Crossref] [PubMed]
- Sharkia M, Michaud S, Berthier MT, et al. Thyroid function from birth to adolescence in Prader-Willi syndrome. J Pediatr 2013;163:800-5. [Crossref] [PubMed]
- Pavone M, Caldarelli V, Khirani S, et al. Sleep disordered breathing in patients with Prader-Willi syndrome: A multicenter study. Pediatr Pulmonol 2015;50:1354-9. [Crossref] [PubMed]
- Lin HY, Lin SP, Lin CC, et al. Polysomnographic characteristics in patients with Prader-Willi syndrome. Pediatr Pulmonol 2007;42:881-7. [Crossref] [PubMed]
- Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366-76. [Crossref] [PubMed]
- Lee CH, Hsu WC, Ko JY, et al. Adenotonsillectomy for the Treatment of Obstructive Sleep Apnea in Children with Prader-Willi Syndrome: A Meta-analysis. Otolaryngol Head Neck Surg 2020;162:168-76. [Crossref] [PubMed]
- Stevenson DA, Anaya TM, Clayton-Smith J, et al. Unexpected death and critical illness in Prader-Willi syndrome: report of ten individuals. Am J Med Genet A 2004;124A:158-64. [Crossref] [PubMed]
- Van Vliet G, Deal CL, Crock PA, et al. Sudden death in growth hormone-treated children with Prader-Willi syndrome. J Pediatr 2004;144:129-31. [Crossref] [PubMed]
- Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, et al. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A 2004;124A:333-8. [Crossref] [PubMed]
- Wilson SS, Cotterill AM, Harris MA. Growth hormone and respiratory compromise in Prader-Willi Syndrome. Arch Dis Child 2006;91:349-50. [Crossref] [PubMed]
- Wolfgram PM, Carrel AL, Allen DB. Long-term effects of recombinant human growth hormone therapy in children with Prader-Willi syndrome. Curr Opin Pediatr 2013;25:509-14. [Crossref] [PubMed]
- Carel JC, Butler G. Safety of recombinant human growth hormone. Endocr Dev 2010;18:40-54. [Crossref] [PubMed]
- Fillion M, Deal C, Van Vliet G. Retrospective study of the potential benefits and adverse events during growth hormone treatment in children with Prader-Willi syndrome. J Pediatr 2009;154:230-3. [Crossref] [PubMed]
- Collett-Solberg PF, Ambler G, Backeljauw PF, et al. Diagnosis, Genetics, and Therapy of Short Stature in Children: A Growth Hormone Research Society International Perspective. Horm Res Paediatr 2019;92:1-14. [Crossref] [PubMed]
- Festen DA, de Lind van Wijngaarden R, van Eekelen M, et al. Randomized controlled GH trial: effects on anthropometry, body composition and body proportions in a large group of children with Prader-Willi syndrome. Clin Endocrinol (Oxf) 2008;69:443-51. [Crossref] [PubMed]
- Miller JL, Shuster J, Theriaque D, et al. Sleep disordered breathing in infants with Prader-Willi syndrome during the first 6 weeks of growth hormone therapy: a pilot study. J Clin Sleep Med 2009;5:448-53. [Crossref] [PubMed]
- Al-Saleh S, Al-Naimi A, Hamilton J, et al. Longitudinal evaluation of sleep-disordered breathing in children with Prader-Willi Syndrome during 2 years of growth hormone therapy. J Pediatr 2013;162:263-8.e1. [Crossref] [PubMed]
- Zimmermann M, Laemmer C, Woelfle J, et al. Sleep-Disordered Breathing in Children with Prader-Willi Syndrome in Relation to Growth Hormone Therapy Onset. Horm Res Paediatr 2020;93:85-93. [Crossref] [PubMed]
- Caudri D, Nixon GM, Nielsen A, et al. Sleep-disordered breathing in Australian children with Prader-Willi syndrome following initiation of growth hormone therapy. J Paediatr Child Health 2022;58:248-55. [Crossref] [PubMed]
- Kang KT, Chou CH, Weng WC, et al. Associations between adenotonsillar hypertrophy, age, and obesity in children with obstructive sleep apnea. PLoS One 2013;8:e78666. [Crossref] [PubMed]
- Ghergan A, Coupaye M, Leu-Semenescu S, et al. Prevalence and Phenotype of Sleep Disorders in 60 Adults With Prader-Willi Syndrome. Sleep 2017; [Crossref] [PubMed]
- Shukur HH, Hussain-Alkhateeb L, Farholt S, et al. Effects of Growth Hormone Treatment on Sleep-Related Parameters in Adults With Prader-Willi Syndrome. J Clin Endocrinol Metab 2021;106:e3634-43. [Crossref] [PubMed]
- Frixou M, Vlek D, Lucas-Herald AK, et al. The use of growth hormone therapy in adults with Prader-Willi syndrome: A systematic review. Clin Endocrinol (Oxf) 2021;94:645-55. [Crossref] [PubMed]
- Heksch R, Kamboj M, Anglin K, et al. Review of Prader-Willi syndrome: the endocrine approach. Transl Pediatr 2017;6:274-85. [Crossref] [PubMed]
Cite this article as: Com G, Santhanam H, Ergun-Longmire B. Respiratory system abnormalities in Prader-Willi syndrome: a literature review. Pediatr Med 2023;6:14.