
Abdominal compartment syndrome as a complication of percutaneous nephrolithotomy requiring extra-corporeal life support—a case report
Introduction
Percutaneous nephrolithotomy (PCNL) is an established therapy for paediatric urolithiasis and is generally safe and well tolerated. PCNL is recommended for stones in the upper ureter or renal pelvis >2 cm, lower pole calyx stones >1 cm, staghorn calculi and when extracorporeal shockwave lithotripsy (ESWL) has been unsuccessful (1,2).
Stone clearance rates in children have improved with the development of new techniques and smaller instruments. PCNL in children is technically more challenging than in adults because of small and hypermobile kidneys which are highly vascular. Although minor complications like fever are common, major side effects are rare.
Abdominal compartment syndrome (ACS) is a rare complication which can arise during PCNL secondary to large volume irrigation fluid leak/extravasation and can be fatal if not detected and treated early. We report a case of irrigation fluid leak and severe ACS during PCNL, progressing to resistant shock and multiple organ failure resulting in death despite maximum intensive care therapy including the use of ECMO. We present the following case in accordance with the CARE reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-94/rc).
Case presentation
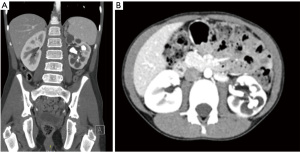
A previously fit & well 10-year-old boy presented to the emergency department with haematuria secondary to a fall. Computerised tomography (CT) abdomen revealed an incidental finding of a left sided staghorn renal calculus extending into the left pelvicalyceal system and the proximal ureter (Figure 1A,1B).
MAG-3 scan revealed the split renal function as right kidney 84% and left kidney 16%. Following further investigation with renal ultrasound scan (Figure 2) and multidisciplinary review he was scheduled for percutaneous nephrolithotomy (PCNL) electively.
Pre-assessment on the day of surgery was unremarkable. Following uneventful inhalational induction of anaesthesia and intubation the patient was initially placed in the lithotomy position to facilitate cystoscopy and perform retrograde studies to assess renal anatomy and subsequently changed to prone position for the PCNL with careful consideration of the pressure areas.
The procedure was technically challenging owing to very complex anatomy. Using ultrasound guidance two punctures were performed of the upper pole and nephrostogram showed a compound calyx draining into the pelvis via a convoluted path (Figure 3A). In our experience puncturing the upper pole allows access to more calyces once a sheath is in situ, whereas with lower pole punctures the pelvic bone can restrict how much the camera or sheath can be manipulated restricting access to all calyces. A Terumo© hydrophilic guidewire and BMC (Boston Medical Centre) catheter were used to try to negotiate a path into the pelvis and ureter, but these were unsuccessful.
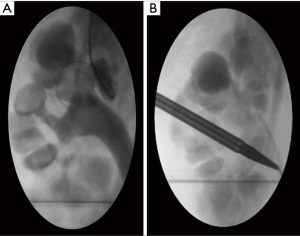
Two further punctures were then performed into calyces in the lower pole. Again, nephrostogram showed compound calyces draining into the pelvis via a collateral path. Retrograde contrast injection from the ureteric catheter was then performed which showed a mid-pole calyx draining directly into the pelvis. This was punctured under fluoroscopic guidance and a Terumo© hydrophilic guidewire negotiated into the pelvis and down the ureter. This was then exchanged for a stiffer wire (Amplatz Super Stiff©), followed by serial dilatation under fluoroscopic guidance to accommodate a 16 Fr sheath (Figure 3B). Under normal circumstances this stage of the procedure takes our experienced interventional radiologist only around 30 min, however due to the complex anatomy this lasted 2–3 h.
Given considerable time was spent trying to gain access, a decision was made to stop after breaking down two to three stones with several stones remaining due to the risks of continuing as the procedure had already lasted almost 5 h. During the procedure there was displacement of the Amplatz sheath once or twice as the renal cortex tissue was very thin and dysplastic. On each occasion the sheath was pushed back into place under direct vision. In total approximately 10 litres of irrigation fluid was used. Following the PCNL, a nephrostomy was placed. Vital signs and ventilator pressures had remained stable throughout the procedure apart from slight rise in heart rate during nephrostomy insertion, which responded to analgesia. However, on completion and removal of drapes the patient was turned supine and was found to have a firm and distended abdomen, rectal prolapse, and his legs were mottled and poorly perfused.
Urgent ultrasound of the abdomen revealed large volume retroperitoneal collection pushing the bladder and bowels anteriorly. Septated fluid was seen around both kidneys and Morison’s pouch, with normal appearances of the liver, spleen and right kidney. The left kidney was decompressed but still had multiple stones. The aorta and inferior vena cava (IVC) were patent. There were bilateral pleural effusions (left bigger than right). Despite a catheter and ureteric stent being in place intraoperatively to enable irrigation fluid entering the kidney to pass down to the bladder and out into the urinary catheter bag, we suspect a proportion of the irrigation fluid was lost from around the Amplatz sheath entry point, as well as the puncture sites made to gain access to the kidney. An 8.5 Fr drain was inserted via the right iliac fossa into the large retroperitoneal collection under ultrasound guidance, draining 1.3 litres of clear fluid. A left pleural drain was also sited and 300 mL blood-tinged fluid was aspirated.
At this point the patient became hypotensive necessitating fluid resuscitation and phenylephrine boluses. Blood gas analysis showed metabolic acidosis with pH 7.1 and lactate 11. A central venous catheter and arterial line were sited, and the patient was transferred to the paediatric intensive care unit (PICU) where inotropes were escalated including dopamine, adrenaline and noradrenaline infusions. There was ongoing blood loss from the abdominal drain despite multiple blood products and an intravenous tranexamic acid infusion, prompting an exploratory laparotomy 4 h following admission to PICU. There was active bleeding and a large clot around the left kidney necessitating a left nephrectomy.
Following readmission to PICU he had ongoing significant bleeding from the abdominal drains and needed high levels of inotropic support. A re-look laparotomy revealed clots in the left paraspinal region/renal bed and bleeding around the left lower ribs, most likely from the intercostal vessels. The exact bleeding points were unidentifiable, so large abdominal wound packs were inserted. Given the context and ongoing concerns about bleeding, the abdomen was left open, and the patient returned to PICU. He developed systemic inflammatory response syndrome (SIRS) which was thought to be due to the combination of reperfusion injury, and ongoing bleeding in spite of multiple blood products. There was multiorgan failure with raised alanine aminotransferase (ALT), deranged clotting and low platelets suggestive of disseminated intravascular coagulation (DIC), lactic acidosis, and raised urea and creatinine with associated oliguria in keeping with acute kidney injury (see Table 1). He was started on empirical broad-spectrum antibiotic therapy with vancomycin and piperacillin-tazobactam. Urine culture grew coliforms sensitive to piperacillin-tazobactam and all blood cultures were negative.
Table 1
| Blood parameter | Immediately postoperative | Postoperative period | |||
|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | ||
| Haemoglobin (g/L) | 112 | 127 | 100 | 95 | 86 |
| White cell count (10−9/L) | 14.72 | 8.6 | 10.04 | 11.18 | 18.48 |
| Neutrophils (10−9/L) | 9.55 | 7.35 | 8.55 | 9.97 | 15.78 |
| Lymphocytes (10−9/L) | 4.53 | 0.99 | 1.14 | 0.86 | 1.66 |
| Platelets (10−9/L) | 164 | 61 | 79 | 41 | 66 |
| PT (s) | 23 | 22 | 27 | 38 | 42 |
| INR | 2 | 1.9 | 2.3 | 3.3 | 3.6 |
| APTT (s) | 58.4 | 45.6 | 42.4 | 46.5 | 58.4 |
| Fibrinogen (g/L) | 0.6 | 1.0 | 1.1 | 0.9 | 0.7 |
| Urea (mmol/L) | 7.4 | 8.2 | 9.4 | 8.8 | 7.7 |
| Creatinine (μmol/L) | 98 | 176 | 204 | 191 | 219 |
| Albumin (g/L) | 22 | 27 | 29 | 33 | 23 |
| ALT (IU/L) | 1,461 | 1,140 | 2,954 | 3,150 | 3,010 |
| Bilirubin (μmol/L) | 21 | 25 | 85 | 115 | 107 |
| Lactate (mmol/L) | 8.7 | 10 | 13 | 11 | 15 |
PT, prothrombin time; INR, international normalised ratio; APTT, activated partial thromboplastin time; ALT, alanine aminotransferase.
He remained severely hypotensive despite significant doses of noradrenaline, adrenaline, vasopressin and hydrocortisone. Echocardiogram showed hyperdynamic left ventricular function with mildly impaired right ventricular filling and function, which improved after draining the right pleural effusion. Unfortunately, there was relentless progression of the shock despite maximal medical therapy including continuous renal replacement therapy (CRRT) and there were signs of compromised perfusion to the peripheries because of the combination of DIC and high dose vasoconstrictors threatening limb viability.
Following multidisciplinary team (MDT) discussion and parental consent, peripheral extracorporeal membrane oxygenation (ECMO) was initiated on PICU to improve organ perfusion, with arterial inflow via a vascular graft to the right femoral artery and return from the right internal jugular vein, and flows of 100–120 mL/kg.
Once on ECMO the inotropic support was reduced and initially the blood pressure was adequate. However, over the next few hours the patient showed increasing periods of bradycardia and intermittent asystole with worsening hypotension despite good ECMO flows. There were no signs of ischemia on electrocardiogram. Vasoactive infusions were re-started, but with no response.
The patient was transferred to the cardiac catheter lab where an atrial septostomy was performed to decompress the left heart that had become distended during periods of asystole. A transvenous pacing system was inserted. Haemodynamic measurements showed high left and right atrial pressures with low ascending aortic mean pressure suggesting low organ perfusion pressures.
Immediately prior to return to PICU, the patient developed fixed dilated pupils. The haemodynamic findings and neurological deterioration were conveyed to the family, following which it was agreed that further active treatment was futile, and he died peacefully surrounded by them.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the parents for the anonymised case details to be published and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
To our knowledge, this is the most severe ACS case described in children undergoing PCNL complicated by irrigation fluid leak. Unfortunately, the complication was fatal despite extensive pharmacological and also ECMO support. This is equivalent to a grade V PCNL complication as per the modified Clavien classification system (1-3) and to our knowledge this is the first time extracorporeal support has been used in this context. The severity and the silent nature of the ACS without any preceding signs deserves further discussion.
PCNL in children is technically more challenging than in adults, with increased risk of vascular damage resulting in bleeding and accidental sheath displacement (2,4). Complications occur in up to 83% of patients but serious complications such as sepsis, bowel and colon injuries are extremely rare (1,2). There is paucity of paediatric case reports; however, irrigation fluid leak is well described as a complication of PCNL in adults. The risk of extravasation increases if the renal pelvis is perforated, or if the parenchyma is already weak due to inflammation from either the stone itself or infection. Fluid leak during PCNL into the retroperitoneum has been reported sparsely, and predominantly in extended adult cases. Intraperitoneal extravasation is rare and quite difficult to detect due to the prone positioning of the patient. Anaesthetists therefore need to watch carefully for subtle signs such as increased diastolic BP and increased ventilator requirements (4). If supracostal percutaneous renal access is necessary due to stone location, there is a risk of hydrothorax which is usually asymptomatic but can occasionally require drainage (2). Complications are more common when performing PCNL for staghorn calculi, owing to the fact they are larger and more complex meaning longer operation times and multiple access points (1).
With fluid extravasation there will usually be some changes in haemodynamics or ventilation, which alerts the anaesthetist to the possibility of ACS. However, in this case there were no signs except a marginal drop in blood pressure and tachycardia just prior to finishing. The prone position and opaque drapes further restricted vision and access leading to delayed recognition of ACS. Accurate measurement of irrigation fluid balance was challenging owing to spillage onto the floor and drapes. The compromised lower limb perfusion and metabolic acidosis with lactate of >10 indicated that the ACS was severe and had been silently evolving for some time.
The only case report that is very similar to our case in terms of the severity, lactic acidosis and development of DIC was by Peterson et al. (5) where a previously healthy young adult male died following ACS and massive haemorrhage.
Pugach et al. (6) reported massive hydrothorax and abdominal distension in a 4-year-old boy following PCNL. This case had early intraoperative ventilation difficulties requiring higher pressures and increased oxygen. Due to early detection of ACS, prompt diagnostic laparoscopy and drainage of 1 litre of fluid the patient made a full recovery.
Poudyal et al. & Etemadian et al. (7,8) reported similar cases in adult patients who were found to have a markedly distended abdomen with high intra-abdominal pressure when turned supine following PCNL. Features of ACS in these patients included hypotension, coagulopathy, lactic acidaemia, acute kidney injury and bleeding. Both recovered following drainage of extravasated fluid and supportive treatment. Tao et al. (9) reported two cases of ACS in adult patients. In both the first clinical sign intraoperatively was a rise in ventilator pressure requirements and in both cases following drainage the patients made a full recovery.
The need for prone position is thought to be contributory to late detection of ACS. There are small case series of supine PCNL with good success and comparable complication rates (10).
Ongoing bleeding, reperfusion injury and the inflammatory cascade contributed to the resistant nature of shock in our patient. The decision to deploy ECMO was very challenging, especially the rationale and timing of ECMO in the absence of available literature or previous use in this context. The aim of ECMO was to improve the cardiac output in order to optimise organ perfusion giving the body time to recover from the profound shock and SIRS.
ECMO has been successfully used in patients with refractory septic shock (RSS). There is conflicting evidence regarding use of ECMO in RSS depending on age, underlying pathology causing the sepsis, timing of ECMO and subgroup selection, as well as optimal mode of ECMO (central versus peripheral ECMO cannulation) (11-13). MacLaren et al. (12) were the pioneers of ECMO use in children in RSS, reporting 47% survival to hospital discharge with peripheral veno-arterial (VA) ECMO use. Another study by the same authors reported improved outcomes with central VA ECMO (13). The majority of their patients had at least 2 or more organ failure, and a third of these had cardiac arrest and were put onto ECMO during cardiopulmonary resuscitation (ECPR). Almost 75% survived to hospital discharge.
The failure of ECMO to help stabilise haemodynamics in our case could be related to many factors. Firstly, the timing of ECMO initiation could have been earlier with better chances of optimal oxygen delivery and shock reversal. Han et al. (14) reported survival of 20% in adults on ECMO for RSS with higher mortality in worse organ failure and longer shock to ECMO duration. Park et al. and Huang et al. (15,16) reported similarly poor survival rates in adults commenced on ECMO for RSS. The evolution of shock in our case was prolonged with established multi-organ failure, and the time from shock onset to ECMO cannulation was almost 48 h.
Secondly, our patient had distributive shock rather than myocardial dysfunction and studies suggest that ECMO is less successful in this subgroup. Bréchot et al. (17) have reported hospital discharge rates of 71% in adults with RSS and severe myocardial dysfunction.
Finally, central ECMO cannulation and higher ECMO flows of 150–200 mL/kg have been reported to be more successful in improving outcomes in RSS (12). Oberender et al. (18) reported that ECMO itself doesn’t improve survival significantly compared to conventional treatment in RSS however it can improve survival and outcomes in a subgroup that suffer cardiac arrest. They also advocate higher ECMO flows (double conventional flows) for better survival. Recently Solé et al. (19) have reported ECMO use in paediatric and neonatal RSS with 33% survival in paediatric patients. Longer hours of sepsis evolution and higher airway pressure prior to ECMO use were seen in non-survivors. In our patient with peripheral ECMO cannulation we were able to achieve flows up to 120 mL/kg so it is unlikely that central VA ECMO would have changed the outcome.
Conclusions
ACS secondary to irrigation fluid leak during PCNL can develop and progress silently without any clinical alerts and can be life threatening. A risk-based approach with proactive monitoring and close communication between the surgeons and anaesthetists is vital. Consideration should be given to accurate measurement of irrigation fluid balance during PCNL especially in children, with regular abdominal checks with or without on-table ultrasound to further reduce the likelihood of this dreaded complication. The role of ECMO for refractory shock in this context should be explored further.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-94/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-94/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-94/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the parents for the anonymised case details to be published and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ozden E, Mercimek MN. Percutaneous nephrolithotomy in pediatric age group: Assessment of effectiveness and complications. World J Nephrol 2016;5:84-9. [Crossref] [PubMed]
- Ganpule AP, Mishra S, Desai MR. Percutaneous nephrolithotomy for pediatric urolithiasis. Indian J Urol 2010;26:549-54. [Crossref] [PubMed]
- Tefekli A, Ali Karadag M, Tepeler K, et al. Classification of percutaneous nephrolithotomy complications using the modified clavien grading system: looking for a standard. Eur Urol 2008;53:184-90. [Crossref] [PubMed]
- Oğuz Ural, Demirelli Erhan, Unsal Ali. Percutaneous Nephrolithotomy in Children. European Medical Journal 2014;
- Peterson GN, Krieger JN, Glauber DT. Anaesthetic experience with percutaneous lithotripsy. A review of potential and actual complications. Anaesthesia 1985;40:460-4. [Crossref] [PubMed]
- Pugach JL, Moore RG, Parra RO, et al. Massive hydrothorax and hydro-abdomen complicating percutaneous nephrolithotomy. J Urol 1999;162:2110-discussion 2110-1. [Crossref] [PubMed]
- Poudyal S, Rai B, Dhital P, et al. Abdominal Compartment Syndrome: A Rare but Fatal Complication of Percutaneous Nephrolithotomy. Asian Journal of Research and Reports in Urology 2018;1:1-5.
- Etemadian M, Shadpour P, Haghighi R, et al. A rare, but life-threatening complication of percutaneous nephrolithotomy: massive intra-abdominal extravasation of irrigation fluid. Urol J 2012;9:614-6. [PubMed]
- Tao J, Sheng L, Zhang HJ, et al. Acute Abdominal Compartment Syndrome as a Complication of Percutaneous Nephrolithotomy: Two Cases Reports and Literature Review. Urol Case Rep 2016;8:12-4. [Crossref] [PubMed]
- Nerli RB, Mungarwadi A, Ghagane SC, et al. Supine percutaneous nephrolithotomy in children. J Sci Soc 2018;45:63-6. [Crossref]
- Ro SK, Kim WK, Lim JY, et al. Extracorporeal life support for adults with refractory septic shock. J Thorac Cardiovasc Surg 2018;156:1104-9.e1. [Crossref] [PubMed]
- Maclaren G, Butt W, Best D, et al. Extracorporeal membrane oxygenation for refractory septic shock in children: one institution's experience. Pediatr Crit Care Med 2007;8:447-51. [Crossref] [PubMed]
- MacLaren G, Butt W, Best D, et al. Central extracorporeal membrane oxygenation for refractory pediatric septic shock. Pediatr Crit Care Med 2011;12:133-6. [Crossref] [PubMed]
- Han L, Zhang Y, Zhang Y, et al. Risk factors for refractory septic shock treated with VA ECMO. Ann Transl Med 2019;7:476. [Crossref] [PubMed]
- Park TK, Yang JH, Jeon K, et al. Extracorporeal membrane oxygenation for refractory septic shock in adults. Eur J Cardiothorac Surg 2015;47:e68-74. [Crossref] [PubMed]
- Huang CT, Tsai YJ, Tsai PR, et al. Extracorporeal membrane oxygenation resuscitation in adult patients with refractory septic shock. J Thorac Cardiovasc Surg 2013;146:1041-6. [Crossref] [PubMed]
- Bréchot N, Luyt CE, Schmidt M, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med 2013;41:1616-26. [Crossref] [PubMed]
- Oberender F, Ganeshalingham A, Fortenberry JD, et al. Venoarterial Extracorporeal Membrane Oxygenation Versus Conventional Therapy in Severe Pediatric Septic Shock. Pediatr Crit Care Med 2018;19:965-72. [Crossref] [PubMed]
- Solé A, Jordan I, Bobillo S, et al. Venoarterial extracorporeal membrane oxygenation support for neonatal and pediatric refractory septic shock: more than 15 years of learning. Eur J Pediatr 2018;177:1191-200. [Crossref] [PubMed]
Cite this article as: Finn D, Ashraf J, Tahir N, Devall M, Van Doorn C, Kumar R. Abdominal compartment syndrome as a complication of percutaneous nephrolithotomy requiring extra-corporeal life support—a case report. Pediatr Med 2022;5:32.


