
The Israel Neonatal Network and National Very Low Birth Weight Infant Database
Background
The Israel Neonatal Network (INN) is a voluntary consortium of all neonatal departments in Israel. The Israel National Very Low Birth Weight (VLBW) Infant Database was established in 1995 under the auspices of the INN. The database is located in the Gertner Institute for Epidemiology and Health Policy Research, Tel Hashomer, Israel and all the activities of the database are coordinated and undertaken by the Women and Children’s Health Research Unit of the Gertner Institute (1).
In 1993, the Israel Neonatal Society proposed to the Ministry of Health the establishment of a national database, which would include data on mortality, morbidity and long-term follow-up of VLBW infants, in order to reduce infant mortality rates in Israel, and to identify factors related to mortality, morbidity and adverse long-term development. The Ministry of Health’s National Council for Obstetrics, Neonatology and Genetics supported this proposal and recommended the establishment of a national database for evaluating birth outcomes, mortality and morbidity of VLBW infants, which would serve as a basis for monitoring VLBW infant mortality and mortality rates, the incidence of congenital malformations, preterm birth, morbidity and disability.
Structure and organization
The INN comprises all neonatal departments in Israel (Appendix 1). Since the establishment of the database, the number of departments has ranged from 26 to 29 due to the closure of some maternity hospitals and the establishment of new hospitals. Neonatal intensive care unit (NICU) facilities are provided in all neonatal departments in Israel, except for one small peripheral hospital, and hence all departments are included in the INN and VLBW database. The establishment of the database was undertaken with the support of, and in coordination with the Israel Ministry of Health, thereby facilitating the linkage of the database with the national birth registry and ensuring completeness of the data collected.
The activities of the database are determined by a forum of the directors of all neonatal departments in the INN and coordinated by the clinical director of the database. This forum considers issues related to all activities of the database, determines the population included and any changes in the data variables collected. The database research committee, comprising five senior neonatologists, is appointed by the forum and any member of the INN is entitled to pursue a research project using the national data. The research committee assesses each request and authorizes the study if appropriate.
The Women and Children’s Health Research Unit of the Gertner Institute is responsible for the administration, maintenance and day-to-day operations of the database, for the data collection, data management and collation, and for the production and distribution of annual national and departmental reports. All data analyses for reporting and research purposes are undertaken by the data managers, epidemiologists and statisticians of the Gertner Institute. The activities of the database are funded in part, by the Israel Ministry of Health based on multi-year agreements, with additional logistical support from the Gertner Institute. The INN and individual NICUs are not required to provide any additional funding for the data management, annual reports or research activities of the database.
The Israel National VLBW Infant Database—process
All neonatal departments in Israel participate in the data collection of the Israel National VLBW infant database. Data are reported for all live born infants of birthweight ≤1,500 g, irrespective of the gestational age at birth. In 2021, the database was expanded to include all infants of ≤31 weeks’ gestation, irrespective of birthweight. The minimal data set was determined upon the establishment of the database, and includes parental demographic details, maternal pregnancy history, treatments and complications, antenatal care, details of the delivery, infant’s status at delivery, infant’s diagnoses, procedures and complications during hospitalization and outcome at discharge (2,3). All departments use an operating manual with standard definitions, which were determined by the INN scientific committee before data collection started and have remained unaltered since. In 2021, the database was expanded to include additional neonatal diagnoses and therapeutic interventions.
All live-born infants in Israel receive a unique identification number at birth. Data are reported using either paper-based or electronic case report forms (CRF). Patient information received by the database coordinator is crosschecked with the national birth registry, provided by the Ministry of Health, and data from any missing infants are requested from the birth hospital. Each CRF is entered into the national database. The reports are examined for missing data and logic errors, including for example, out of range measurements. If necessary, the participating center’s database coordinator is contacted for clarification and further elaboration. The infant’s identification number facilitates the acquisition of additional information related to infant transfers to other hospitals or to another department within the birth hospital and data are collected on all infants until death or discharge home. An annual data file is prepared for each participating hospital and subsequently, birth hospital and patient identification remain confidential. From 1995 through 2019, the database includes reports on 39,000 VLBW infants and 33,000 mothers reflecting over 99.5% of all VLBW live births in Israel.
Objectives
“There are three principal means of acquiring knowledge … observation of nature, reflection, and experimentation. Observation collects facts; reflection combines them; experimentation verifies the result of that combination” (Denis Diderot, French author, encyclopedist & philosopher, 1713–1784).
The aim of the database is to identify perinatal conditions related to mortality, morbidity and disability among VLBW infants and to support national programs and initiatives aimed to reduce infant mortality rates and improve outcomes. The main objectives, delineated upon the establishment of the database, were: the application of quality data for the assessment of morbidity and mortality trends of VLBW infants (4,5); benchmarking of individual neonatal unit performance in comparison to national data; quality of care and management; planning of national, regional and institutional structure and policy development; longitudinal developmental assessment and for collaborative research programs.
Database reporting activities
The predominant activity of the database relates to data collection, collation and presentation of the data in unique annual reports for each of the participating NICUs and in an annual national report presented to departmental, institutional and governmental health care leaders.
Annual departmental report
Each neonatal department receives annual reports that include frequencies and distributions of all data reported for the specific department and for the national data. Percentile ranks compared to the national median and quartile ranges, are reported for approximately 60 selected items related to demographic characteristics, pregnancy complications, infant morbidities and treatments (Figure 1). The reports provide the individual departments with a benchmark in relation to the national results; however, by consensual agreement of the INN, comparative data for the departments are not reported.
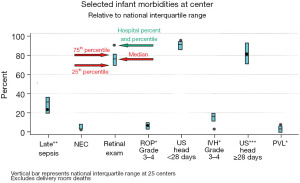
Annual national report
The national annual report includes maternal and infant data for the complete VLBW population, data by birthweight and gestational age groups and 5-year trends for process and outcome measures. This report is distributed extensively to senior governmental and institutional healthcare executives. The annual report includes a supplementary analysis on a subject identified by the database team, which may be of interest to policy makers and stakeholders. Some examples of these reports include: post discharge mortality among VLBW infant (6); infertility treatment and rates of multiple births; impact of reducing the number of triplet births on NICU bed requirements; mortality and morbidity among Arab VLBW infants (7); perinatal factors associated with infant mortality (to 1 year) by age of death; trends in length of hospitalization in NICUs; risk factors and estimation tool for mortality among extremely premature infants (8).
The database also provides information or data analyses requested by the Ministry of Health or governmental institutions including for example, the Office of State Comptroller, the Parliamentary (Knesset) Research Committee, The National Council for Obstetrics, Neonatology and Genetics and others.
Research
The database provides a research platform for all participants in the INN. The INN research committee considers all research proposals submitted, based especially on the scientific validity of the research question and the availability of data to address the proposed study, whilst assuring the confidentiality of participating units. All approved study proposals are submitted to the institutional review board (Helsinki committee) of the Sheba Medical Center. The database clinical director and team of data managers, epidemiologists and statisticians of the Gertner Institute for Epidemiology and Health Policy Research assist in designing the study, statistical analysis and data interpretation, and with manuscript preparation.
The first INN study published in 2001 (2), evaluated the relationship between periventricular/intraventricular hemorrhage and antenatal exposure to tocolytic treatment. Since then, approximately 60 studies have been published in leading pediatric, obstetric and perinatal medical journals, in addition to over 20 studies in association with the International Network for Evaluating Outcomes in Neonates (iNeo), collaboration (9). More than 60 neonatologists, obstetricians and epidemiologists from 15 different hospitals and organizations have undertaken studies in the context of the database.
The database files include the patient’s unique identification number thereby enabling linkage with national or institutional databases. The linked files are subsequently de-identified and the de-identified secure linked files serve as the research file for the proposed study. Some examples of linked studies include: an assessment of post-discharge infant mortality applying data from the national birth and deaths registry (6); rehospitalization of VLBW infants through childhood and adolescence in linkage with the Maccabi Health care services electronic patient records (10); and the association between postnatal steroid therapy and autism spectrum disorder in VLBW infants also in linkage with the Maccabi Health care services patient records (11).
International collaboration
In 2012, the INN was invited to participate in the establishment of the International Network for Evaluating Outcomes in neonates. iNeo is an international collaboration that includes population-based national neonatal networks of Australia and New Zealand, Canada, Finland, Israel, Italy, Japan, Spain, Sweden, Switzerland and the UK (9). The main aim in forming iNeo was to provide a powerful platform for applied health services and policy research that can be used to improve patient-oriented outcomes for VLBW infants in the member countries and globally. The INN has actively participated in the ongoing activities and in the 23 publications to date of iNeo, providing interesting and useful comparative cross-country information on the management and outcomes of VLBW infants. The iNeo collaboration has provided the INN with important comparisons with the other collaborating networks of both outcome data and management processes (12,13). This collaboration has also supported our active participation in the Canadian Neonatal Networks’ Evidence-based Practice for Improving Quality (EPIQ) program, providing guidance and tools for the establishment of local quality initiatives.
Quality improvement (QI)
The Israel Neonatal Society representing all neonatal departments has promoted, initiated and coordinated QI programs encompassing all INN collaborators. The QI initiative aims to promote the development of a network that will train QI teams and support QI projects in perinatology, at national and local levels. The activities of the network include: training teams to carry out QI processes through workshops and advanced training; defining and initiating projects to improve quality at the national level defining goals and indices; developing the projects’ toolbox; simulation training and encouraging “horizontal” learning (benchmarking). In general, the QI initiatives are initiated by the committee of the Israel Neonatal Society, in coordination with the heads of all neonatal departments.
The INN has initiated two major QI programs and although voluntary, all departments have elected to participate in the programs:
- A national program to reduce both central line associated blood stream infections (CLABSI) and all episodes of blood stream infection (BSI) in NICUs (termed “Touch Zero”);
- A national program to reduce severe intra-ventricular hemorrhage (SIVH) among neonates delivered between 24- and 28-week gestational age (termed “Protect my Brain”).
“Touch Zero” QI initiative
The INN has over the years, reported excessively high rates of late onset sepsis among VLBW infants (14). In 2013 Israeli NICU CLABSI rates were significantly higher than many international peers: the VLBW infant cohort’s rate of ~7 CLABSIs/1,000 line days compared unfavorably with USA’s rates of ~1 CLABSI/1,000 line days. Wide variability in rates in these homogenous VLBW populations suggested that factors other than common biologic risk contributed to the observed differences. The goal of the “Touch Zero” program was to reduce the neonatal BSI rates in VLBW infants by 25% for each of two successive years from the baseline measure. A project committee was established in early 2014 to lead and manage the effort. The committee addressed gaining stakeholders’ buy-in (including national and hospital leaders, each NICU’s medical/nursing leaders and staff members); identifying and modifying nursing and medical “best” practices; developing a dissemination strategy (semi-annual face-to-face meetings, supplemented by monthly telephone conference calls, tool kits, checklists, critical care process audits); training in QI methods; systematic reflection on infections; and data reporting and feedback processes (collaborative data collection, analysis and feedback, linkage with Infection Control Department staff). Baseline data were provided by the Ministry of Health’s National Institute for Infection Control and Antibiotic Resistance. De-identified aggregate data were collected, submitted and managed monthly by each NICU.
Bundles distributed in June 2014 addressed hand hygiene, central and peripheral line insertion, line set-up and maintenance, BSI investigation and feedback and included 48 specific recommendations related to policy and procedure, equipment and materials, and training and organizational effectiveness. Collaborative meetings emphasized NICU presentations of challenges with and techniques for adopting recommendations, as a means to emphasizing the sharing dimension of collaborative membership.
This initiative has resulted in a significant decrease in VLBW CLABSI and BSI. Total BSI rates decreased by 43% from the baseline of 3.1/1,000 to 1.8/1,000 patient days, in 2017 (Figure 2). The VLBW CLABSI rate fell by 60% from 7.8/1,000 to 3.0/1,000 central line days over the same period. The national VLBW database has concomitantly recorded a decrease of 45% in the percent of VLBW infants with episodes of late onset sepsis, from 20.9% in 2010–2014 to 11.4% in the period 2015–2019.
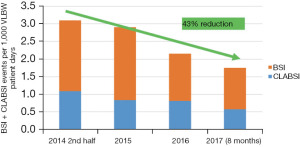
“Protect my Brain” QI initiative
The rates of SIVH among VLBW preterm infants in Israel were reportedly significantly higher than in international peers (12,15). The aim of the “Protect my Brain” QI initiative was to reduce the SIVH rate in premature infants delivered between 24- and 28-week gestation by 25% for each of two successive years from the baseline measure. Successful reductions in individual NICU SIVH rates have been reported but network QI interventions have had variable results (16,17). An expert committee established for this project comprised neonatologists, obstetricians, neonatal nurses and midwives. Hospital QI teams were created and national meetings supplemented by monthly telephone conferences and procedures for the collection of care process and outcome data instituted. Interventions focusing on practices during the infant’s first 100 hours of life included 14 clinical care bundles relating to antepartum management, delivery room processes and NICU processes. Some examples are the development of an extended neonatal resuscitation (NRP) scenario and video training program to improve teamwork and evaluation processes, simulation-based NRP training modules, the administration of “rescue” antenatal corticosteroid therapy, delayed cord clamping, optimizing infant admission temperature, infant ventilation practices, minimal handling and more. As the SIVH prevention recommendations are complex in range and scope of practice, additional time and effort has been provided for the currently ongoing preparation and team training activities. The INN hopes to be able to assess and quantify the impact of this initiative in the coming year.
Present challenges and future plans
The ongoing and continued operation of the VLBW infant database faces multiple challenges. Data collection is voluntary and as demands increase, the ability to collect an expanded dataset is becoming more and more difficult. In most neonatal departments, senior neonatologists undertake the data collection. To ensure optimal and timely collection of high-quality data a trained data administrator is required for each NICU, however at present there is no provision of funding for data coordinators in the NICUs by either the Ministry of Health or by the individual hospitals. To date the database includes information on VLBW infants only. Recently, the reported population was expanded to incorporate all live born infants of ≤31 weeks’ gestation and we have yet to assess the compliance of the individual units and the completeness of data collection for this population. Further expansion to include larger neonates with defined morbidities such as early sepsis, malformations and neonatal encephalopathy has been recommended, but will be contingent on the provision of adequate infrastructure and staffing for an expanded database.
The instigation of QI programs has been extremely challenging for the INN. Teams do not have enough knowledge, experience or adequate training in advancing QI processes and there is a concomitant lack of qualified personnel for managing the programs. The consensual agreement by INN collaborators not to report comparative data from the NICUs has impeded the development of a structured benchmarking process as an important aspect of the QI initiatives. The INN is planning the development of policies and procedures necessary for the promotion of QI projects. The initiative aims to institute a QI network that will train teams and support QI projects in perinatology at national and local levels. This process includes the establishment of a steering committee to determine and prioritize the QI projects and to promote workshops for the training of medical staff to lead QI processes at local and national levels. Further upgrading of the VLBW infant database and dataset is required in order to provide timely and easily accessible data in support of the quality programs.
Finally, the expansion of the database to include long-term outcome data of VLBW and preterm infants is of prime importance to the network. An Israel Ministry of Health directive has determined the framework for the community follow-up and management of “complex” and preterm infants after hospital discharge (Medical Administration Directive 20/16; 15/11/2016). The infants’ community health care provider and pediatrician are responsible for undertaking the defined follow-up program including neurodevelopmental, growth and nutritional assessments, and referral for any further intervention as required. The directive however, does not include any provision for the collection, integration or reporting of outcome data at a national level. At present long-term outcome measurements are unfortunately not being collected at a national level and are not included in the VLBW infant database. A research project linking the VLBW database with one of the community health care provider’s electronic patient record is presently being pursued, in order to assess the long-term health and developmental outcomes of these infants who comprise approximately 25% of VLBW infants in Israel.
Summary
The Israel National VLBW Infant Database was established in 1995 under the auspices of the INN, a voluntary consortium of all neonatal departments in Israel. For over 25 years, the database has provided all the collaborating NICU’s with annual reports comparing the individual units’ measures to the national median and interquartile percentiles, in order to assist in identifying problem areas. Data provided to the Ministry of Health and other governmental institutions have been instrumental in supporting and advancing neonatal care and neonatal care facilities in Israel. The database has provided a rich and comprehensive source of data for research, undertaken by INN collaborators and the decade-long participation in the iNeo collaboration has highlighted significant challenges and areas for improvement. In recent years, the INN under the auspice of the Israel Neonatal Society has promoted, initiated and coordinated QI programs. The future activities of the INN and the database will aim to promote additional quality initiatives and support the further development and improvement of neonatal care in Israel.
Acknowledgments
Funding: The Israel National Very Low Birth Weight Infant Database is partially funded by the Israel Ministry of Health.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Shoo Lee and Prakesh Shah) for the series “Neonatal Networks for Outcomes Improvement: Evolution, Progress and Future” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-69/coif). The series “Neonatal Networks for Outcomes Improvement: Evolution, Progress and Future” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Levitzky O, Lerner-Geva L, Dollberg S, et al. The Israel National Very Low Birth Weight Infant Database. Harefuah 2016;155:32-6, 67. [PubMed]
- Weintraub Z, Solovechick M, Reichman B, et al. Effect of maternal tocolysis on the incidence of severe periventricular/intraventricular haemorrhage in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2001;85:F13-7. [Crossref] [PubMed]
- Riskin A, Riskin-Mashiah S, Lusky A, et al. The relationship between delivery mode and mortality in very low birthweight singleton vertex-presenting infants. BJOG 2004;111:1365-71. [Crossref] [PubMed]
- Grisaru-Granovsky S, Reichman B, Lerner-Geva L, et al. Population-based trends in mortality and neonatal morbidities among singleton, very preterm, very low birth weight infants over 16 years. Early Hum Dev 2014;90:821-7. [Crossref] [PubMed]
- Grisaru-Granovsky S, Boyko V, Lerner-Geva L, et al. The mortality of very low birth weight infants: the benefit and relative impact of changes in population and therapeutic variables. J Matern Fetal Neonatal Med 2019;32:2443-51. [Crossref] [PubMed]
- Kugelman A, Reichman B, Chistyakov I, et al. Postdischarge infant mortality among very low birth weight infants: a population-based study. Pediatrics 2007;120:e788-94. [Crossref] [PubMed]
- Dollberg S, Mimouni FB, Lusky A, et al. Effect of ethnicity on mortality of very low birthweight infants in Israel. Arch Dis Child Fetal Neonatal Ed 2003;88:F333-8. [Crossref] [PubMed]
- Bader D, Kugelman A, Boyko V, et al. Risk factors and estimation tool for death among extremely premature infants: a national study. Pediatrics 2010;125:696-703. [Crossref] [PubMed]
- Shah PS, Lee SK, Lui K, et al. The International Network for Evaluating Outcomes of very low birth weight, very preterm neonates (iNeo): a protocol for collaborative comparisons of international health services for quality improvement in neonatal care. BMC Pediatr 2014;14:110. [Crossref] [PubMed]
- Kuint J, Lerner-Geva L, Chodick G, et al. Rehospitalization Through Childhood and Adolescence: Association with Neonatal Morbidities in Infants of Very Low Birth Weight. J Pediatr 2017;188:135-141.e2. [Crossref] [PubMed]
- Davidovitch M, Kuint J, Lerner-Geva L, et al. Postnatal steroid therapy is associated with autism spectrum disorder in children and adolescents of very low birth weight infants. Pediatr Res 2020;87:1045-51. [Crossref] [PubMed]
- Shah PS, Lui K, Sjörs G, et al. Neonatal Outcomes of Very Low Birth Weight and Very Preterm Neonates: An International Comparison. J Pediatr 2016;177:144-152.e6. [Crossref] [PubMed]
- Lui K, Lee SK, Kusuda S, et al. Trends in Outcomes for Neonates Born Very Preterm and Very Low Birth Weight in 11 High-Income Countries. J Pediatr 2019;215:32-40.e14. Erratum in: J Pediatr 2021 Jun;233:294-299. [Crossref] [PubMed]
- Makhoul IR, Sujov P, Smolkin T, et al. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 2002;109:34-9. [Crossref] [PubMed]
- Gemmell L, Martin L, Murphy KE, et al. Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks' gestation. J Perinatol 2016;36:1067-72. [Crossref] [PubMed]
- Lapcharoensap W, Bennett MV, Powers RJ, et al. Effects of delivery room quality improvement on premature infant outcomes. J Perinatol 2017;37:349-54. [Crossref] [PubMed]
- McLendon D, Check J, Carteaux P, et al. Implementation of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Pediatrics 2003;111:e497-503. [Crossref] [PubMed]
Cite this article as: Reichman B, Klinger G, Zangen S, Levitzki O, Lerner-Geva L; in collaboration with the Israel Neonatal Network. The Israel Neonatal Network and National Very Low Birth Weight Infant Database. Pediatr Med 2023;6:20.

