
Identification of sick children in acute care settings
Introduction
“Spotting the Sick Child” is a core component of clinical practice for those working in any setting where children are assessed and managed. The aim of this paper is to create a knowledge synthesis focusing on the recognition of the sick child and, where felt to be relevant or controversy in the evidence base, their very early management. This is neither a vade mecum or definitive bibliography more a summation of common principles across common topics to guide both junior and experienced staff in their clinical practice and teaching. The focus of the paper is on the selection of patients who need further observation, investigation and management. The article focuses on infants and children, rather than young people, given these are the largest consumers of acute healthcare and present the largest risk of missed diagnosis. Given the huge scope of the topic resuscitative management is not addressed, as explained in the article, the most critically unwell patients are the easiest to recognise. However, we discuss how the definition of ‘sick’ is an imprecise term and therefore cover a range of potential presentations that require active cognition on the part of the clinician.
Methods
The authors, all members of the Don’t Forget the Bubbles (DFTB) team, drew collectively upon the published works of DFTB, eight years of blog posts covering all aspects of paediatric emergency medicine supported by evidence-based medicine and research. These articles were combined to create a ‘meta-blog’, a concise collective summery of multiple blogposts allowing the reader to gain a concise overview prior to commencing their own, deeper learning through articles provided on the DFTB website.
How do clinicians recognise ‘the sick child’?
The term ‘spotting the sick child’ is synonymous with paediatric practice and at virtually every medical school, children’s hospital and continuing professional development course there will be teaching on this topic. However, despite the ubiquity of the term, there is much to unpick from these four words. Specifically, what ‘spotting’ actually entails and what is the definition of ‘sick’? The art of paediatrics involves the amalgamation of what you hear (the history) and what you see (the examination).
What you hear
The history, whether told by a Parent and Carer (henceforth Guardian) or the child themselves, is a narrative account in essence of concerns around a specific problem or problems. The ability to listen to not only the factual account but the tone with which it is delivered is vital. Guardian concern, much like clinician gut feeling (which will be covered shortly), is a poorly defined concept but one which is repeatedly found to be dismissed or not recognised in reviews of the literature. In essence it is the demonstration of unease about the particular state a child presents with. It is a challenging concept as almost by proxy the child being brought for medical attention would imply concern. However the vernacular is probably more correctly (although anecdotally) associated with concern that can’t be addressed by simple explanation or investigation. A guardian may be worried about their child’s swollen arm, which is alleviated by demonstration of a fracture on x-ray and the administration of a plaster cast. The concern for their child may remain in the context of being worried about their pain or time to recover but now the source of the problem has been identified the Guardian is happy to take them home. However the child with a fever may, in the eyes of the clinician, have an obvious source such as an otitis media. Despite an explanation, guardian concern may persist as the guardian doesn’t believe their child is ‘right’. This may be due to misplaced anxiety about the impact of fever or it may be recognition their child is truly not acting in a normal fashion. The former is a common occurrence due to fever phobia, however to dismiss the latter risks discharging a child who does indeed have a serious bacterial infection. This balance represents a significant challenge, especially for the junior clinician. This is because the problem is compounded by the fact guardian concern is not only directly verbally expressed, it may also be inferred by body language or response to questions. A hierarchy exists between the healthcare professional and guardian and some may feel unable to challenge this. Recognising this imbalance and addressing concern is one of the first phases of being able to spot the sick child. And counter-intuitively, one in which the actual clinical condition of the child is for practical purposes irrelevant.
What you see
The term gut feeling is an oft-quoted, “I knew this child wasn’t right, my gut feeling told me so”. But like guardian concern this is a term without a good definition. A child with low saturations, poor perfusion and decreased consciousness is extremely unwell. It is irrelevant what the cause is, whether this turns out to be influenza or streptococcal pneumonia, intervention is mandated. Gut feeling is not telling you this child is unwell, their clinical signs are. And hence lies the conundrum in ‘spotting’ the sick child. The most unwell children are obviously unwell. They can be recognised by the public with no medical training and are a group who are rarely mismanaged. They are similar to the obviously well, the child who is running around a waiting room, smiling and eating a packet of crisps. Both groups are not a matter of debate in relation to their recognition or outcome.
However gut feeling may be usefully applied to the child who perhaps has been unwell for a couple of days, has a fever, perhaps a slightly raised respiratory rate or heart rate. There is no obvious focus save bilaterally red drums (which may just be local peripheral vasodilation often inappropriately ascribed as otitis media). The treating clinician is faced with an impossible task, one in which guidance retrospectively may appear coherent but at the time is fraught with inconsistency. Does this child need screening for sepsis due to a risk of infection (which they have due to the fever) and a tachycardia (a completely normal response to the fever and distress of an unfamiliar environment)? In some localities there can be prompt review by an experienced clinician whose ‘gut feeling’ will be to confirm this is indeed most likely to be a viral infection. However not all services have access to a paediatrician or equivalent and it may be that blood tests and other investigations are undertaken. Encapsulating what it is about a child’s appearance that makes them decide to investigate or not investigate, treat or not treat, has proved extremely difficult to determine. Current sepsis screening criteria do not help as softer physiological signs are poorly specific; recent retrospective and prospective observational studies have determined less than 1% of children presenting this way will have a serious bacterial infection (1,2). And gut feeling has not been demonstrated to be a useful metric. In a prospective study of febrile infants less than 90 days old (3) clinician prediction of the presence of serious bacterial illness (SBI) was extremely poor. In this study 1/5 (21.4%) of infants who actually had a SBI were felt to have had a less than 1% risk of doing so (prior to investigations or treatment) by the treating clinician. Conversely of those infants in which the clinician thought there was a greater than 50% chance of having an SBI only 11% actually did (3).
The terms gut feeling and gestalt are sometimes used interchangeably but clinical gestalt is a gut feeling derived from pattern recognition. The clinician’s mind is an incredible machine, but clinical gestalt needs time to develop, honed from multiple patient encounters. Prediction tools can support clinicians, both while developing clinical intuition and to support recognition of conditions not frequently encountered. For example the Petechiae in Children (PIC) study (Figure 1), published in 2021, looked to assess the performance of eight regional and national clinical guidelines in identifying children with invasive meningococcal disease (5). In total, 1,334 children under 18 presenting to 37 emergency departments in the UK with fever above 38 ℃ and non-blanching rash or features suggestive of meningococcal infection were included. Nineteen (1%) had meningococcal disease. Eleven (2%) children were admitted to the paediatric intensive care unit (PICU), eight of whom had N. meningitidis. An additional seven children had non-meningococcal invasive bacterial infection (IBI), with other bacterial pathogens including pneumococcus, E. coli and Group A Streptococcus.

How did the guidelines do? All eight guidelines had a sensitivity of 100%, identifying all 26 cases of IBI. Specificity was much lower however, varying greatly between guidelines. The National Institute for Health and Care Excellence (NICE) sepsis guideline had the lowest specificity of zero; every patient was stratified as being high risk. The NICE meningitis guideline had the second lowest specificity at 1%. Low specificity meant that children without the disease were investigated and treated, at a cost of £660.41 per patient. The Barts Health NHS Trust guideline had the highest specificity at 36%, and therefore lowest cost per patient of £490.29.
How about clinical gestalt? The guidelines were adhered to in 46% of patients. Deviation resulted in fewer antibiotics being given and therefore a lower cost per patient. Two children who were discharged had early meningococcal disease; both were subsequently treated and neither needed admission to PICU. Clinical gestalt had a higher specificity than the guidelines (fewer children without meningococcal disease or an IBI were treated with antibiotics). This was at the cost of sensitivity, with a clinical gestalt sensitivity of 89%.
A further challenge is in the definition of ‘sick’. As previously highlighted there are a collection of children who are sick as defined by having an infection requiring treatment. However their presenting features may not be characteristic of a child with florid symptoms. This has been repeatedly proven by the number of serious case reviews where children are discharged only to return with profound sepsis or in cardiac arrest. In these cases, it is more a case of spotting the children with developing infection. Efforts to spot the sick child must also acknowledge Baye’s theorem in which the prevalence of disease plays a significant role in its detection. Vaccine preventable infectious diseases are (relatively) rare and therefore clinical exam or biomarkers may do little to increase their post-test probability. For example, meningococcal disease has a dramatically lower incidence meaning that the presence of a petechial rash and fever is far less likely to signify the presence of an invasive organism. In fact, the relative sparsity of infectious diseases means that developing infections following medical review is just as likely as having the disease in the first place. In a striking editorial ‘sick children are sick’, Green et al. (6) highlight that perhaps we are probably only able to spot children who have clear features of decompensation and that the majority of children who return to healthcare settings with invasive disease probably didn’t have it at the first presentation.
There is a low baseline rate of significant bacterial infection in the immunised child population of the developed world. To discharge a child safely and comfortably though, we require agreement between clinician and guardian that the child is safe for discharge and that the guardian understands that deterioration is possible and what that deterioration would look like. This can be difficult to achieve with a snapshot view, and indeed when the child is febrile and tachycardic—features which typically wax and wane in viral disease. Just like a video can give more information than a still photograph, watching a child’s progress over a period of time can give more information than assessment at a single point in time. Consideration of geography, family logistics and the mechanics of the healthcare system, as well as the natural history of the suspected pathology, are required when discharging a child. Different healthcare environments have different capabilities for observation, investigation and review. For the vast majority of unwell children, discharge into guardian care with robust safety netting advice is appropriate. Where there are concerns about the likelihood or potential speed of deterioration, guardian understanding of the signs of deterioration or an inability of the family to return to the healthcare facility for geographical or logistical reasons, ongoing observation should be provided. In the emergency department context, time-based targets make this observation challenging and mandate that our systems are set up to support this with short stay wards, paediatric assessment units or low barriers to access in-patient care. “Mum is still worried” is an acceptable reason to keep a child in hospital.
So how does one ‘spot the sick child’? Probably with great difficulty. The clinician must be aware of prior risk of disease, must listen carefully to the guardians and pay meticulous attention to clinical examination. This article subsequently breaks down presentation into key areas starting with the sick neonate.
Spotting the sick neonate
The neonatal period is a time of great transition, consisting of the first 28 days of life, during which the human body undergoes remarkable changes (7). When presented with the possibility of an unwell baby, there are a few key diagnoses that the assessing clinician should consider (Figure 2).

Firstly, is this baby septic? Sepsis is the most common cause of neonatal illness, can present insidiously, and can have disastrous consequences including death, if missed (9). Respiratory disease comes a close second, with conditions such as bronchiolitis. Thought should be given to the possibility of an underlying cardiac or metabolic disorder. It should be stressed that a ‘sick neonate’ can be a presentation of non-accidental injury, so this should always be considered if other, more obvious, causes are not present.
Neonatal sepsis
When assessing the potentially sick neonate, a focused history is vital. Features such as the presence of maternal Group-B Streptococcus, prolonged rupture of membranes or maternal fever will heighten the concern for sepsis. A thorough feeding history, including whether they are waking for feeds and volumes and type of feed is important. Weight gain is a good sign the baby has been feeding well but doesn’t necessarily preclude the presence of sepsis.
Septic babies can fall anywhere between moribund and ‘something’s not quite right.’ The NICE developed a traffic light system (Table 1) for assessing fever in the under-five age range which highlights that a fever greater than 38 ℃ in infants under three months is a ‘red-flag’ risk for sepsis (10).
Table 1
| Risk factor | Green—low risk | Amber—intermediate risk | Red—high risk |
|---|---|---|---|
| Colour (of skin, lips or tongue) | Normal colour | Pallor reported by parent/carer | Pale/mottled/ashen/blue |
| Activity | Responds normally to social cues | Not responding normally to social cues | No response to social cues |
| Content/smiles | No smile | Appears ill to a healthcare professional | |
| Stays awake or awakens quickly | Wakes only with prolonged stimulation | Does not wake or if roused does not stay awake | |
| Strong normal cry/not crying | Decreased activity | Weak, high-pitched or continuous cry | |
| Respiratory | Nasal flaring | Grunting | |
| Tachypnoea: respiratory rate | Tachypnoea: respiratory rate >60 breaths/minute | ||
| • >50 breaths/minute, age 6–12 months; | Moderate or severe chest indrawing | ||
| • >40 breaths/minute, age >12 months | |||
| Oxygen saturation ≤95% in air | |||
| Crackles in the chest | |||
| Circulation and hydration | Normal skin and eyes | Tachycardia: | Reduced skin turgor |
| Moist mucous membrane | • >160 beats/minute, age <12 months | ||
| • >150 beats/minute, age 12–24 months | |||
| • >140 beats/minute, age 2–5 years | |||
| Capillary refill time ≥3 seconds | |||
| Dry mucous membranes | |||
| Poor feeding in infants | |||
| Reduced urine output | |||
| Other | None of the amber or red symptoms or signs | Age 3–6 months, temperature ≥39 ℃ | Age <3 months, temperature ≥38 ℃ |
| Fever for ≥5 days | Non-blanching rash | ||
| Rigors | Bulging fontanelle | ||
| Swelling of a limb or joint | Neck stiffness | ||
| Non-weight bearing limb/not using an extremity | Status epilepticus | ||
| Focal neurological signs | |||
| Focal seizures |
Practice varies considerably in terms of investigation, with the Rochester criteria (11) shaping current paediatric practice. However, criticism that, serious bacterial infection rates were low in their patient population, with the subsequent potential for over-investigation, led to the development of newer algorithms such as the Step-by-step criteria (10). Step-by-Step’s sensitivity of 92% and negative predictive value of 99.3%, versus Rochester’s 81.6% and 98.3% for IBI and non-IBI in the ‘low risk’ group, supports its use as a tool for determining likelihood of IBI in neonates (11). The PECARN criteria (3), although not yet externally validated, are a useful aid to decision making with excellent sensitivity and negative predictive values to rule out serious bacterial infections. However, the availability of serum procalcitonin measurements may create a limiting factor in their application. Sepsis remains a leading cause of death in neonates and all decision-making tools acknowledge the importance of clinical judgement in determining which neonates will require treatment.
Cardiac issues
Congenital heart disease (CHD) is the most common congenital abnormality with incidence of 4–10 per 1,000 live births (12). Babies presenting collapsed with cyanosis, particularly cyanosis unresponsive to supplemental oxygen, should always have CHD considered as a differential (Figure 3).
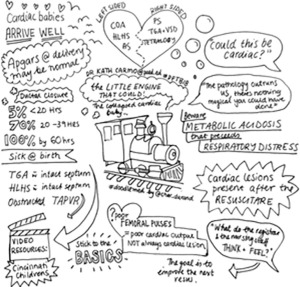
Neonatal heart failure presents with poor feeding, tachycardia, tachypnoea and sometimes hepatomegaly. There may be weak or absent femoral pulses and a murmur may or may not be present.
A difference of >10% in pre- and post-ductal oxygen saturations can be a useful screening tool, and most babies in the UK, Australia and New Zealand, will now have this performed prior to discharge from hospital. Although there is a false positive rate of 0.14%, research has shown pulse oximetry aids early diagnosis of CHD (14-16).
The diagnosis can be supported if a chest X-ray shows cardiomegaly, hyperaemic or oligaemic lung fields and if the baby remains hypoxic after being placed on 100% oxygen for 10 minutes, the hyperoxia test (17).
Treatment consists of prostaglandin (alprostadil in the UK at 10–100 nanogram/kg/min) whenever a duct-dependent lesion is suspected. Critically unstable neonates should be intubated, aiming for oxygen saturations of 75–85%. Prostaglandin side-effects include apnoea therefore intubation may also be needed at higher doses.
Echocardiogram is required for definitive diagnosis so be prepared to transfer the baby, if needed, to a cardiac centre.
Metabolic problems
Inborn errors of metabolism (IEM) can be difficult to diagnose in babies, with similar presentations to the above conditions. Whilst individually IEM are rare, incidence can rise as high as 1:800 live births when considered as a collective whole (18,19). Broadly speaking metabolic disorders can be classified as intoxication, energy metabolism, or complex molecule disorders depending on the affected pathway (20).
Clinician suspicion for IEM should be raised in the neonate presenting with persistent hypoglycaemia, a metabolic acidosis or a raised ammonia level >100 mmol/L (20). The presence of a metabolic acidosis on venous or capillary blood gas, should prompt the clinician to calculate the anion gap to help narrow down the diagnosis (Figures 4,5).
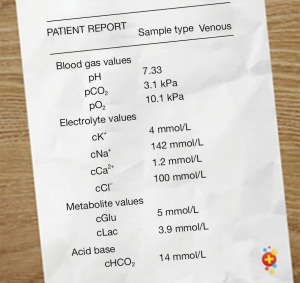

As sepsis is always our first consideration, treatment with broad-spectrum antibiotics is indicated. In addition, specific treatments include stopping the source of protein (stopping feeds), avoiding catabolism (giving intravenous glucose 10–15 mg/kg/min 10% dextrose) and rehydration/resuscitation with intravenous fluids (22).
Hypoglycaemia should be corrected as per APLS guidelines with 2 mL/kg of IV 10% dextrose (23).
Ammonia levels should be checked and sent on ice to the laboratory without delay. Local and national guidance varies but levels >100 mmol/L should usually prompt discussion with a metabolic team.
The child with breathing difficulties
Paediatric respiratory presentations are common, particularly during winter months. Although the majority have only mild respiratory disease, children are prone to respiratory decompensation. With the high volume of presentations of a heterogenous group of conditions, how do we spot the sick child with a respiratory complaint? And why is it that children are at such risk of respiratory decompensation?
Children have compliant chest walls and horizontal ribs, making the signs of respiratory distress relatively easy to identify (Table 2). Children with respiratory distress tire quickly and, without intervention, are at risk of respiratory failure because of both their anatomical differences and their respiratory physiology. Their metabolic rates are high with a high oxygen consumption and the high percentage of respiratory muscle fast twitch fibres tire easily, together resulting in rapid hypoxia.
Table 2
| Sign | Physiological and anatomical reason |
|---|---|
| Tachypnoea | Compliant chest walls and horizontal ribs limit the ability to increase tidal volume and so respiratory rate increases to compensate |
| Head bobbing in young infants | Use of accessory neck muscles pulls the infant’s head forward on inspiration |
| Nasal flaring | Enlarging the nostrils decreases resistance to air flow |
| Tracheal tug | Combination of accessory neck muscle use and increased diaphragmatic movement pull the trachea down during inspiration against neck muscles in contraction |
| Sternal recession | Increased negative intrathoracic pressures pull the sternum in during inspiration |
| Intercostal recession | The soft, compliant chest wall and increased intrathoracic negative pressures mean the soft tissues between the ribs draw in on inspiration |
| Subcostal recession and abdominal breathing |
Weak respiratory muscles lead to a reliance on the diaphragm and abdominal muscles to increase the volume in the intrathoracic cavity, leading to indrawing below the ribs and obvious movement of the abdomen during breathing |
| Grunting | Breathing out against a closed glottis increases intrathoracic pressure to improve gas exchange and prevent the small airways collapsing during expiration |
Infection is the most common cause of paediatric respiratory distress. On face value, this seems like a simple statement but paediatric respiratory infections are a heterogenous group of conditions that can present in similar ways. Imagine a 15-month-old with a history of cough, coryza and fever, whose guardian is concerned about the extent of the child’s respiratory distress. Differentials could include upper respiratory tract infections ranging from mild tonsillitis to life-threatening retropharyngeal abscess. Differentials could also include lower respiratory tract infections such as bronchiolitis, viral induced wheeze or pneumonia. All these conditions have the potential to become life-threatening; all have different treatment approaches. Recognition of the critically unwell child with a respiratory presentation is not difficult. What is more challenging is differentiating between the aetiologies of moderate respiratory distress and instigating the correct management to prevent potential deterioration given children’s unique respiratory physiology.
Imagine the 15-month-old has signs of severe respiratory distress: tachycardia, tachypnoea, hypoxia at 91% saturation in air, tracheal tug and intercostal and subcostal recession with abdominal breathing. They look pale and tired. Auscultation of their chest reveals wheeze. By exploring two common, similar but pathophysiologically distinct paediatric respiratory infections, it is evident that correctly diagnosing and therefore correctly managing ‘the sick respiratory child’ can be a challenge.
The presence of wheeze may imply bronchospasm from a condition common in toddlers and preschool aged children, descriptively termed viral induced wheeze. The pathophysiology is similar to that of a viral exacerbation of asthma, without other features of reactive airway disease. Viral induced wheeze typically starts with symptoms of an upper respiratory tract infection. Much like in acute asthma, the mainstay of the treatment is to reverse bronchospasm, with inhaled or nebulised bronchodilators such as salbutamol acting as a smooth muscle relaxant. Like asthma, escalation of care in more severe cases is to intravenous bronchodilators such as magnesium sulphate, salbutamol and aminophylline, although currently there is no evidence to suggest one therapy is superior to another (24). Unlike asthma, the role of steroids in preschool wheeze is less clear. A widely cited study by Panickar et al. in 2009 (25) found no benefit of steroids in this age group, but the inclusion of infants as young as 10 months led many to question Panickar’s findings. A more recent study by Foster et al. (26) in 2018 suggested a role for oral steroids in wheezy children between 2 and 6 years of age, but only if severe enough to require hospital admission, and not if atopic (contrary to previous thoughts). Further evidence in favour of steroids in preschoolers was found by Wallace et al. in their 2021 (27) randomised controlled study. What studies like these suggest is that there are likely to be different phenotypes of wheeze in preschool aged children with different pathophysiological causes, some that will respond to steroids and some that will not.
The 15-month-old is initiated on treatment for viral induced wheeze; saturations improve with oxygen but despite inhaled bronchodilators, there is no other improvement. Steroids are given, an intravenous cannula is sited and intravenous bronchodilators are given. There is still no improvement. The child is beginning to look tired. The question arises: does this child need further escalation of care or could this be a different disease entity?
Bronchiolitis is the most common cause of hospital admission in children in their first year of life. It can present up to 24 months of age and often has a similar presentation to viral induced wheeze. Correct diagnosis, and therefore implementation of the correct management, can be particularly challenging in children between 12 and 24 months of age, because of these two phenotypically similar but pathophysiologically distinct diseases. Like viral induced wheeze, bronchiolitis is a viral infection. However, instead of bronchospasm of the larger airways, bronchiolitis, as the name implies, is a disease of the bronchioles. Bronchiole inflammation results in luminal narrowing, exacerbated by intra-lumenal secretions. Narrowed bronchioles result in wheezing sounds on expiration, while air bubbling through secretions can cause fine inspiratory crepitations. Auscultation findings can be dynamic, with predominantly wheeze, predominantly crepitations or a combination of the two, changing at any given time. Degree of respiratory distress is very variable, peaking between days 3 and 5 of illness, with certain risk factors for a more severe disease course (Table 3).
Table 3
| Younger age, particularly under 6 weeks |
| Congenital heart disease |
| Neurological conditions |
| Chronic respiratory illness |
| Pulmonary hypertension |
| Ex-premature infants |
| Inborn errors of metabolism |
| Trisomy 21 |
| Immunodeficiency |
| A previous severe bronchiolitis illness requiring non-invasive ventilation or PICU admission |
PICU, paediatric intensive care unit.
Despite huge drives in research to find a treatment for bronchiolitis, a recent systematic review by the PREDICT network has found no role for bronchodilators, antibiotics, steroids or nebulised adrenaline and only weak evidence for nebulised hypertonic saline (28). Treatment remains supportive, with oxygen, non-invasive ventilation, or intubation to support ventilation and either nasogastric feeds or intravenous fluids to maintain hydration. The role of high flow nasal cannula oxygen (HFNC), the delivery of humidified, heated oxygen at high flow rates, has been of recent interest. A second systematic review by the PREDICT network did not show any evidence for the use of HFNC use in infants with bronchiolitis without hypoxia or as an early treatment (29). The authors’ recommendations are to reserve HFNC if there is a deterioration after starting standard nasal cannula oxygen in infants with hypoxia.
Although the 15-month-old child with wheeze and respiratory distress can be a diagnostic challenge, spotting the ‘sick’ child with a respiratory presentation is less challenging. Targeting interventions, or indeed no interventions bar supportive care, to match the likely aetiology to prevent respiratory fatigue is the mainstay of treatment.
The child with a head injury
The challenge in managing a child after a head injury is deciding whether or not they are at risk of a clinically important brain injury (ciTBI), ‘clinically important’ being one that requires neurosurgical intervention, more than 24 hours intubation, two nights’ stay in hospital or death. If the risk is high, then it is imperative that intracranial haemorrhage is detected early so that neurosurgical intervention can be facilitated in a timely manner. Computerised tomography (CT) is the imaging modality of choice but comes with a small but significant exposure to ionising radiation, and with it the small, but definite, risk of a future malignancy. The challenge to clinicians is deciding which children are at significant enough risk of a ciTBI to warrant the radiation exposure from CT. At what point does the risk of radiation outweigh the risk of missing a significant brain injury? This is where the question ‘can we spot the sick child’ (or in this case, the child with a ciTBI) is raised. Identifying children with significant risk factors is arguably not that challenging. The child with a reduced Glasgow Coma Scale (GCS) below 14, or a persistently reduced GCS that sits at 14 for some time after the injury is clearly at risk of a significant injury. That child warrants a CT. The child with signs of a base of skull fracture (raccoon eyes, bruising behind the ears, bleeding behind the tympanic membrane, or CSF leaking from the ears or nose) is also easy to spot, as is the child under two years with a palpable skull fracture. These children will also need a CT. The child with worsening symptoms, or persistent signs of an altered mental state will also clearly need imaging as the risk of a ciTBI without resolution of symptoms is too high to ignore. But what about the child with, what we’ll call, intermediate risk factors for ciTBI? In the absence of a severe risk factor but other, softer signs of intracranial embarrassment, when do we decide to organise a scan?
Findings from secondary analysis of the Australasian APHIRST study, suggest that experienced clinicians are good at identifying children with a ciTBI with high sensitivity of 98.8% and high sensitivity of 92.4% (30). Their sensitivity is similar to three paediatric head injury clinical decision rules, PECARN, CATCH and CHALICE, but with a lower CT rate. This might present the argument that spotting the child with a ciTBI is not that challenging. However there is a definite role for clinical decision rules to guide less experienced clinicians in identifying which children to scan.
Subsequent to the APHIRST study, PREDICT developed a clinical decision rule for use in Australia and New Zealand (31). PREDICT adapted the risk criteria from the PECARN rule (32), identified as the best performing rule in the APHIRST study, supported by a literature search to find the best available evidence for managing children with additional underlying conditions.
Those softer, intermediate, risk factors (Table 4) are clearly identified in the PREDICT rule, which recommends that when two intermediate risk factors are present, a senior clinician can choose to either image with CT or actively observe the child to monitor for a change in the signs or symptoms. This concept of planned observation is important; it is incorporated into the PECARN rule and was shown by PREDICT to be associated with significantly lower CT rates than prescriptively following the decision rules, without increasing the risk of clinically important traumatic brain injury.
Table 4
| Post-traumatic seizure |
| Severe mechanism of injury (motor vehicle accident with patient ejection or rollover, death of another passenger, pedestrian or cyclist without helmet struck by motor vehicle, falls of ≥1 m in <2 years, fall >1.5 m in ≥2 years, head struck by high impact object) |
| History of loss of consciousness |
| Abnormal neurological examination |
| Severe headache |
| History of vomiting in children ≥2 years |
| Non-frontal haematoma in children <2 years |
In addition to standard risk factors, PREDICT have included evidence-based and consensus-derived guidance for thresholds for CT in children with other conditions. With guidance on when to scan children with ventriculo-peritoneal shunts, children with bleeding disorders or on anticoagulant therapy, a neurodevelopmental disorder, possible abusive head trauma or intoxication, this guideline helps clinicians adjust their scanning thresholds based on the best available evidence.
Abdominal pain
Abdominal pain is another common paediatric presentation. Many children will have relatively insignificant or self-limiting conditions such as gastroenteritis, mesenteric adenitis or constipation. However, there are several not-to-be-missed diagnoses in paediatrics that are in the forefront of our minds, plus a few more that we probably don’t think about enough.
Before thinking about specifics, a general impression of the child is useful; and this is the first stage in spotting the sick child. As with any assessment of a child, it’s really useful to be able to have a look at the child and the way they’re behaving and moving (or not moving) before they see you. If you’re calling a child out of the playhouse in the waiting room, you’ll feel better than if they’re lying on a trolley, or across the waiting room chairs. If they’re able to walk in, what do they look like? Have they got a normal gait or are they doubled over like an elderly person or walking like John Wayne? (in this latter case, always check the testes). If they’re not already on a trolley, are they able to climb up by themselves? For small children to climb onto a trolley takes a good bit of effort and movement; if they can do this unaided, it’s generally reassuring. Make it into a game if the child needs some encouragement: “Do you think you can climb all the way up there on your own?”.
After this general impression, comes the history. This is the time to listen out for some particular nuggets of information that will guide your investigations and differential diagnosis. The ‘speed bump sign’ is a well-known experience-based pointer to a potential diagnosis of appendicitis. Guardians will describe the child being in significant pain at every bump in the road on the journey to hospital. More evidence-based are symptoms of fever, pain that migrates to the right iliac fossa and vomiting (33).
Examination can be tricky in young children, and this can lead to a delay in identifying important clinical signs. Strategies such as lying the child across the guardian’s lap, rather than on the trolley can be helpful, as can making the examination into a game: “I’m going to see if I can guess what you had for breakfast by feeling your tummy”. Certain parts of the examination, such as checking for rebound tenderness can be stressful for young children and may be better tested by asking the child to jump or cough. Throughout the examination, a focus on the face, rather than the abdomen, is helpful, along with a stream of distracting chat (learn a bit about children’s TV, if it’s not already in your skill set). Discouraging the guardian from asking the child to ‘tell the doctor where it hurts’ will also allow you to make a more accurate assessment. Allowing babies to stay on the caregiver’s lap is usually the preferred option, and some non-nutritive sucking can facilitate an easier examination.
Appendicitis is the most common cause of acute abdomen requiring surgery in childhood and carries a lifetime risk of 7–8%. Its highest incidence is in the 10–20-year age group. It presents more of a diagnostic challenge in the younger age group, particularly those under four, where perforation is much more common in up to 80% (34). Symptoms of appendicitis overlap with several other conditions, particularly in the early stages, making diagnosis more of a challenge. Identifying the child who has a perforated appendix is likely to be easy, but picking up the signs earlier in the process presents more of a challenge.
In spotting the sick child, there are several tools that we can use to support our decision making. With specific reference to the diagnosis of appendicitis, one such tool is the Alvarado score (35) (Figure 6) which includes symptoms, signs and laboratory findings which are designed to guide the clinician in management. Other scores exist and include the paediatric appendicitis score (36).

Two systematic reviews concluded that the cutoff point of 5 was good at ruling out appendicitis in children (99% sensitivity); however at a cut-off point of 7 to rule-in appendicitis, the score performed poorly (specificity 64–82%) (37,38). The bottom line is that the Alvarado score is pretty good at ruling out appendicitis in those with a score of <5, but it’s likely to overestimate the likelihood of appendicitis in those with intermediate or higher scores. For the emergency clinician, this tool could therefore be used as part of the decision-making process to help rule out appendicitis. The very fact that you’re calculating the Alvarado score means that you’ve taken blood and this, in itself will naturally give rise to a period of observation while the results are awaited. During this time, the natural progress of the condition can be monitored with serial observations and the ability to get a general sense of whether the child is improving or deteriorating.
Other investigations should include urinalysis. Urinary tract infection is an important differential diagnosis in abdominal pain. A note of caution is that leucocytes and microscopic haematuria is often found in those with appendicitis because an inflamed appendix can irritate the bladder and ureter. Careful history and examination are important in making the diagnosis. Females over the age of about 12 should be consented for pregnancy test as part of the urinalysis.
Ultrasound has a sensitivity of around 87% for appendicitis, with a specificity of around 89%, with a 10–20% chance of non-visualisation of the appendix; CT is more sensitive (91%) and specific (94%) but with risks in terms of radiation exposure (39). CT is not currently widely used for this indication in the UK but is a preferred option in North America.
Intussusception is a particularly concerning diagnosis for the emergency and urgent care clinician. Described as the “great mimicker”, missing this diagnosis is a source of concern for the emergency paediatric clinician. We think of the classic triad of intermittent abdominal pain, palpable abdominal mass and redcurrant jelly stool as being our key clues to a diagnosis of intussusception. However, this triad occurs in only around one third of children. Abdominal pain is the most common symptom across all ages; while in children under 12 months the strongest predictors are vomiting, irritability and redcurrant jelly stool (40).
Many children with intussusception present with vague, intermittent symptoms and guardian reports that the child is ‘just not right’. Careful history taking will often elicit a history of pale, floppy episodes. These can be short-lived and the child is almost back to normal in between. A period of observation can be useful here, but if there is a reasonable level of suspicion, the child should have an ultrasound, as delay in diagnosis is associated with higher chance of failure to reduce the intussusception by air enema. Across the age range, ultrasound performs well at diagnosing intussusception with point of care ultrasound having a sensitivity of 96% and specificity of 92.6% in diagnosing intussusception in one study (41).
Ectopic pregnancy & pelvic inflammatory disease
As paediatric clinicians we need to improve our ability to record a sexual history in young people, both from a psychosocial perspective, and in this case as a possible cause for abdominal pain. While ectopic pregnancy can present with a wider range of symptoms that can resemble more common conditions, it should be suspected in sexually active young people presenting with abdominal or pelvic pain, missed period or vaginal bleeding (with or without clots). Ask about breast tenderness, shoulder tip pain and dizziness or syncope (42). Always undertake a pregnancy test if this is a possibility. Adolescent females can present very unwell with pelvic inflammatory disease and we may not always consider this early enough in this population.
Conclusions
The identification of sick children is challenging and while clinicians are good at identifying really sick children correct diagnosis can be more nuanced in the grey areas. Clinical gestalt is a honed skill, requiring experience as the building blocks of acumen and until that expertise is achieved it is important to utilise clinical practice guidelines and seek help. Often a good outcome for a child and their family may come down to either diligent observation over time and/or robust safety netting of the features of further deterioration.
Acknowledgments
We thank all the contributors to dontforgetthebubbles.com for contributions to the website that have assisted in the production of this article.
Funding: None.
Footnote
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-54/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-54/coif). The authors are members of Don’t Forget the Bubbles. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gomes S, Wood D, Ayis S, et al. Evaluation of a novel approach to recognising community-acquired paediatric sepsis at ED triage by combining an electronic screening algorithm with clinician assessment. Emerg Med J 2021;38:132-8. [Crossref] [PubMed]
- Nijman RG, Jorgensen R, Levin M, et al. Management of Children With Fever at Risk for Pediatric Sepsis: A Prospective Study in Pediatric Emergency Care. Front Pediatr 2020;8:548154. [Crossref] [PubMed]
- Kuppermann N, Dayan PS, Levine DA, et al. A Clinical Prediction Rule to Identify Febrile Infants 60 Days and Younger at Low Risk for Serious Bacterial Infections. JAMA Pediatr 2019;173:342-51. [Crossref] [PubMed]
- Davis T. Petechiae in Children – the PiC Study, Don't Forget the Bubbles, 2020. Available online: https://doi.org/
10.31440/DFTB.30782 - Waterfield T, Maney JA, Fairley D, et al. Validating clinical practice guidelines for the management of children with non-blanching rashes in the UK (PiC): a prospective, multicentre cohort study. Lancet Infect Dis 2021;21:569-77. [Crossref] [PubMed]
- Green SM, Nigrovic LE, Krauss BS. Sick kids look sick. Ann Emerg Med 2015;65:633-5. [Crossref] [PubMed]
- Doherty TM, Hu A, Salik I. Physiology, Neonatal; 2021.
- Pediatric Emergency Playbook (Host) (2015) The Undifferentiated Sick Neonate [Audio podcast epsode] https://pemplaybook.org/podcast/the-undifferentiated-sick-infant/
- Fleischmann C, Reichert F, Cassini A, et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child 2021;106:745-52. [Crossref] [PubMed]
- National Institute for Health and Care Excellece (NICE) Feverish illness in Children Guidance (NG 143); 2019.
- Dagan R, Powell KR, Hall CB, et al. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855-60. [Crossref] [PubMed]
- Gomez B, Mintegi S, Bressan S, et al. Validation of the "Step-by-Step" Approach in the Management of Young Febrile Infants. Pediatrics 2016;138:e20154381. [Crossref] [PubMed]
- Browning Carmo K. The Collapsed Cardiac Child at DFTB18, Don't Forget the Bubbles, 2019. Available online: https://doi.org/
10.31440/DFTB.18978 - Prudhoe S, Abu-Harb M, Richmond S, et al. Neonatal screening for critical cardiovascular anomalies using pulse oximetry. Arch Dis Child Fetal Neonatal Ed 2013;98:F346-50. [Crossref] [PubMed]
- Ewer AK. Pulse oximetry screening for critical congenital heart defects in newborn infants: should it be routine? Arch Dis Child Fetal Neonatal Ed 2014;99:F93-5. [Crossref] [PubMed]
- Thangaratinam S, Brown K, Zamora J, et al. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet 2012;379:2459-64. [Crossref] [PubMed]
- Ossa Galvis MM, Bhakta RT, Tarmahomed A, et al. Cyanotic Heart Disease 2021;
- Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969-1996. Pediatrics 2000;105:e10. [Crossref] [PubMed]
- Sanderson S, Green A, Preece MA, et al. The incidence of inherited metabolic disorders in the West Midlands, UK. Arch Dis Child 2006;91:896-9. [Crossref] [PubMed]
- Saudubray JM, Baumgartner MR, Walter JH. (editors) Inborn Metabolic Diseases. Diagnosis and treatment. 6th Edition. Springer; 2016.
- Alegra T, Metabolic presentations part 1: neonates, Don't Forget the Bubbles, 2020. Available online: https://doi.org/
10.31440/DFTB.28423 - Häberle J, Boddaert N, Burlina A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis 2012;7:32. [Crossref] [PubMed]
- Advanced paediatric life support guidelines version 6e. ALSG. Aug 2019.
- Craig SS, Dalziel SR, Powell CV, et al. Interventions for escalation of therapy for acute exacerbations of asthma in children: an overview of Cochrane Reviews. Cochrane Database Syst Rev 2020;8:CD012977. [Crossref] [PubMed]
- Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med 2009;360:329-38. [Crossref] [PubMed]
- Foster SJ, Cooper MN, Oosterhof S, et al. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2018;6:97-106. [Crossref] [PubMed]
- Wallace A, Sinclair O, Shepherd M, et al. Impact of oral corticosteroids on respiratory outcomes in acute preschool wheeze: a randomised clinical trial. Arch Dis Child 2021;106:339-44. [Crossref] [PubMed]
- O'Brien S, Borland ML, Cotterell E, et al. Australasian bronchiolitis guideline. J Paediatr Child Health 2019;55:42-53. [Crossref] [PubMed]
- O'Brien S, Craig S, Babl FE, et al. 'Rational use of high-flow therapy in infants with bronchiolitis. What do the latest trials tell us?' A Paediatric Research in Emergency Departments International Collaborative perspective. J Paediatr Child Health 2019;55:746-52. [Crossref] [PubMed]
- Babl FE, Oakley E, Dalziel SR, et al. Accuracy of Clinician Practice Compared With Three Head Injury Decision Rules in Children: A Prospective Cohort Study. Ann Emerg Med 2018;71:703-10. [Crossref] [PubMed]
- Babl FE, Tavender E, Dalziel S. On behalf of the Guideline Working Group for the Paediatric Research in Emergency Departments International Collaborative (PREDICT). Australian and New Zealand Guideline for Mild to Moderate Head injuries in Children – Full Guideline. 2021. PREDICT, Melbourne, Australia.
- Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet 2009;374:1160-70. [Crossref] [PubMed]
- Peyvasteh M, Askarpour S, Javaherizadeh H, et al. MODIFIED ALVARADO SCORE IN CHILDREN WITH DIAGNOSIS OF APPENDICITIS. Arq Bras Cir Dig 2017;30:51-2. [Crossref] [PubMed]
- Bundy DG, Byerley JS, Liles EA, et al. Does this child have appendicitis? JAMA 2007;298:438-51. [Crossref] [PubMed]
- Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med 1986;15:557-64. [Crossref] [PubMed]
- Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care 2015;31:164-8. [Crossref] [PubMed]
- Ohle R, O'Reilly F, O'Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med 2011;9:139. [Crossref] [PubMed]
- Heineman J. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. BET 1: An evaluation of the Alvarado score as a diagnostic tool for appendicitis in children. Emerg Med J 2012;29:1013-4. [Crossref] [PubMed]
- Doria AS. Optimizing the role of imaging in appendicitis. Pediatr Radiol 2009;39:S144-8. [Crossref] [PubMed]
- Mandeville K, Chien M, Willyerd FA, et al. Intussusception: clinical presentations and imaging characteristics. Pediatr Emerg Care 2012;28:842-4. [Crossref] [PubMed]
- Trigylidas TE, Hegenbarth MA, Patel L, et al. Pediatric Emergency Medicine Point-of-Care Ultrasound for the Diagnosis of Intussusception. J Emerg Med 2019;57:367-74. [Crossref] [PubMed]
- Webster K, Eadon H, Fishburn S, et al. Ectopic pregnancy and miscarriage: diagnosis and initial management: summary of updated NICE guidance. BMJ 2019;367:l6283. [Crossref] [PubMed]
Cite this article as: Platt R, Priddis K, Lawton B, Hall D, Roland D; Don’t Forget the Bubbles. Identification of sick children in acute care settings. Pediatr Med 2023;6:5.

