
Congenital mitral valve defects in pediatric population: a narrative review of surgical repair
Introduction
The mitral valve (MV) is a complex morphologic and functional apparatus constituted by the annulus, leaflet valves, tendinea chords, papillary muscles, and left ventricular wall (1). Defects affected in any of the components will result in complicated congenital mitral valve malformations (CMVM), including MV stenosis, MV regurgitation, or mixed lesions. CMVM is very rare in the pediatric population, with an incidence of 0.49% (2), and it commonly coexists with other cardiac defects, such as ventricular or atrial septal defect or coarctation of the aorta (2-5). CMVM can cause significant hemodynamic alterations, resulting in pulmonary hypertension, congestive heart failure, and even death, depending on the various pathology, heterogeneous lesions, other severe coexisting cardiac defects, and disease progress. Therefore, patients with CMVM usually need early surgical interventions. The aim of MV repair is not merely for anatomy repair of the MV, but more importantly, for recovery of functional hemodynamic.
Carpentier made the first functional classification in 1976 (subsequently revised in 1998), comprising two main categories based on MV dysfunction: mitral regurgitation and mitral stenosis (6). Since only a few case reports and small series have been described in the pediatric population, and the impact of recent surgical strategies on clinical outcome is mainly unknown, therefore, the present work aimed to provides a detailed review of surgical strategies of CMVM, focusing on the surgical considerations, techniques, and outcomes. We present the following article following the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-52/rc).
Methods
We conducted a literature search using online database PubMed, Web of Science, and Embase with the terms “Congenital mitral valve malformation”, “mitral valve defect”, “mitral valve stenosis”, “mitral valve regurgitation”, “infant”, “pediatric”, “children”, “surgical repair”, “mitral Annuloplasty”, “Mitral valve replacement”, and “surgical outcome” for papers up to Sep, 2021. We also consulted standard textbooks. This review excludes the congenital malformations of MV in atrioventricular septal defect, atrioventricular discordance, and straddling MV (Table 1). We restricted our search to articles in English that were about human beings. We included original research as well as systematic reviews and meta-analysis. The references for critical reviews and frequently-cited primary research publications were also included.
Table 1
| Items | Specification |
|---|---|
| Date of search | From Apr 1, 2021 to Sep 30, 2021 |
| Databases and other sources searched | PubMed, Web of Science, and Embase |
| Search terms used | “Congenital mitral valve malformation”, “mitral valve defect”, “mitral valve stenosis”, “mitral valve regurgitation”, “infant”, “pediatric”, “children”, “surgical repair”, “mitral Annuloplasty”, “Mitral valve replacement”, and “surgical outcome” |
| Timeframe | Literatures published up to Sep, 2021 |
| Inclusion and exclusion criteria | Study type: Original Articles, Systematic Reviews, Meta-Analysis, Standard Textbooks; language restrictions: English |
| Selection process | WC and ML conducted the selection process independently. All authors discussed a standarded literature selection criteria and discussed the selected literature |
Surgical indications and considerations
Severe symptoms and signs of pulmonary hypertension are the definitive indications for surgical intervention. Severe MV regurgitation or stenosis is a definitive surgery indication. Asymptomatic CMVM patients with severe pulmonary hypertension should also be considered for surgery. Another indication of surgery is when moderate MV regurgitation or stenosis coexists with other congenital cardiac lesions (7). CMVM can also be examined and repaired when the other congenital cardiac lesions are treated.
Surgical treatment of CMVM remains a considerable therapeutic challenge, including valve repair or valve replacement. MV repair is preferable. Compared with the valve replacement, mitral valve repair showed several advantages, including conservation of the sub-valvular apparatus, preservation of ventricular geometry, conservation of left ventricular function, maintaining the growth potential of the native annulus, and long-term survival (8). In the pediatric population, valve replacement is less desirable because the mismatch between the native annulus and MV prosthesis is a risk factor for both early and late mortality, and it has a risk of re-operation with child growth (9). MV surgical repair also decreases the risk of thromboembolism and avoids the need for long-term anticoagulation, which is particularly difficult to manage in the pediatric population, especially for female patients.
The CMVM encompasses complex lesions, including anomalies on valvar leaflets, tensor apparatus, and papillary muscles. Therefore, various surgical techniques are tailored for each anomaly, and combinations of several surgical techniques are often required to achieve the primary goal of achieving a suitable valve function, rather than a ‘‘normal’’ anatomy.
MV instrumental evaluations
An accurate preoperative diagnostic assessment is essential for evaluating of anatomical and function of MV and planning individual tailored surgical strategies. Transthoracic two-dimensional echocardiography remains the standard diagnostic technique using in pediatric surgical planning. Recently, the three-dimensional echocardiography technique helps plan the surgery strategy by en face view of the anatomy of valves and chordal, providing better precise measurement of the MV annulus estimation of the entire mitral annulus, mitral area, and regurgitant (10-12). Moreover, it can provide unique views of the sub valvar MV apparatus, such as parachute MV (13). Other assessments are also performed on MV defects, such as Doppler ultrasound and magnetic resonance imaging (14,15).
Besides, intro-operative echocardiographic assessment of MV anatomy and function is also important to evaluate the effectiveness of the procedure. If patients still presented with significant residual mitral regurgitation after the MV repair, revision or the initial repair or mitral valve replacement (MVR) should be considered to get a better outcome and reduce the risk of re-operation (16). Also, intro-echocardiography is used to assess residual cardiac defects and identify unsuspected lesions (17,18).
Surgical repair techniques
Surgical repair techniques in MV regurgitation
Mitral annuloplasty
In children, the expansion and deformation of the valve annulus mainly occur in the posterior annulus, and mitral constriction is the primary mitral annuloplasty surgery. Studies have shown that mitral annuloplasty can restore valve leaflets and correct left ventricular systolic and diastolic function without impairing leaflets motion or reducing the orifice area (19). Mitral annuloplasty retains the maximum valve tissue and sub-valvular tissue. It maintains the ventricular geometry and the normal function of the valve and ventricular, which is conducive to the improvement and recovery of left ventricular function and long-term survival. Pediatric mitral annuloplasty includes modified DeVega or Reed annuloplasty and artificial annulus ring (Carpentier ring). For infants, the modified Kay-Wooler (Figure 1) and the Reed procedures are preferable. For older infants, the modified Paneth (Figure 2) or DeVega procedures are commonly used. Artificial annulus rings can be used in children but not recommended for infants, considering scar formation and restriction of the annulus growth and leaflet activity caused by sutures and artificial valve annulus (19).
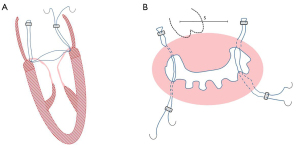
Moreover, studies showed biodegradable ring for CMVM was superior to non-ring annuloplasty, especially for young children with smaller annular sizes when standard commercial rings were not available or recommended (20). Besides, the autologous pericardium is used in annulus constriction, and studies showed this technique preserves mitral annulus stability and flexibility and decreases the risk of mitral stenosis (21-23). Compared with Cor-Matrix, using autologous pericardium in MV repair was associated with a lower inflammatory reaction, more tissue infiltration, remodeling, vascularization, and neointima formation (21). Newly engineering treatment using bovine pericardium conferred outstanding resistance to calcification and preserved more controlling healing than the treated autologous peridium in a growing lamb model, which provided a promising direction for mitral annuloplasty in the future (24).
MV repair
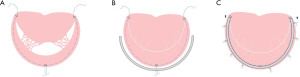
The anterior leaflet MV cleft is closed with interrupted fine stitches, avoiding leaflets distortion and leaflets junction reduction. Meanwhile, an annular plication is usually performed. When the regurgitation was peripheral or near the commissure, the zone of apposition might be closed completely using interrupted stitches. The prolapsing segment was firstly resected in a quadrangular fashion, confirming that both ends of the remnant leaflets had enough thickness and strength. The adjacent leaflet tissue was then detached from the annulus, over approximately a half-length of the resected gap. The two leaflet ends were brought together by compression sutures placed on the annulus and sutured together. Pledgetts were used to reinforce sutures whenever the leaflet tissue was considered fragile. For the sliding leaflet technique, abnormal chordae tendineae are often attached to the free edges of the cleft. In most cases, the cleft can be closed after resection of the abnormal chordae (25). To patients with leaflet tissue defects, extending the leaflet with a biological patch, such as glutaraldehyde treated autologous pericardial or heterologous patch material, is applied to patients (9). Alfieri first proposed MV double orifice valve repair technique, also known as the edge-to-edge technique (Figure 3), which was used as a salvage procedure for patients who had residual regurgitation after attempts with other techniques. The anterior and posterior leaflets were sutured together at the midpoint. This technique is easy to operate and works well with less reoperation and mitral stenosis, showing promising efficacy in mortality, reoperation rates, and complications (26). Even with severe anterior leaflet malformation, mitral double orifice repair and annuloplasty can achieve desirable results. At present, this technique has become a routine surgical treatment of pediatric MV prolapse with incomplete closure (26-29). Percutaneous edge-to-edge MV repair using the MitraClip has been used in children in several cases, and it may prove to be a novel and effective palliation to consider in a subgroup of pediatric patients (30,31).

Sub-valvular structure repair
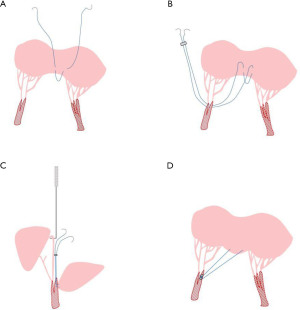
MV chordoplasty (elongation and rupture of the chordae) is an important cause of MV insufficiency. Chordae papillary muscle plasty is commonly performed for sub-valvular structure repair. For MV prolapse caused by the simple lengthening of the chordae, the commonly used method is to shorten the chordae, split the papillary muscles, fold the excessively long part of the chordae and bury them in the papillary muscles (32). For the rupture of the main tendon of the anterior leaflet of the MV or the lack of chordae at the edge of the anterior leaflet, the normal autogenous chordae can be transferred (33). Besides, artificial chordae implantation (Figure 4) are usually applied to elder children in a range of pathological settings with satisfied effectiveness (34-40), with advantages that the number, length, and implantation of the implanted tendons can be determined according to the intro-operation situation. The proper determination of the chordae to meet children’s growing needs is still the main challenge of this technique.
Surgical repair techniques in MV stenosis
Supra-valvar
Supra-annular mitral ring (SMR) is a rare developmental anomaly of the supra-valvular area of the MV, producing various lesions in the left ventricular. Surgical management of SMR has been proven to obtain a good prognosis with appropriate strategies. SMR is commonly associated with other cardiac anomalies, such as ventricular and left ventricular outflow tract pathologies, especially sub-aortic membrane, bicuspid aortic valve, and coarctation of the aorta. And it is one of the specific features of Shone’s complex (41,42). For the supra-mitral ring, attaching above the valve annulus, direct resection can be performed. For the intra-mitral ring where thickened tissue covers the atrial surface of the leaflet and often crosses the commissures, resection can be difficult, especially in young infants because the ring tissue is usually very adherent to the native leaflet. Surgical correction consists of resecting the fibrous tissue, taking care not to damage the anterior leaflet and pulmonary veins.
Valvar
The restricted leaflet mobility is usually caused by fibroelastic tissue growth as a membrane on the leaflets themselves or a thick chord, like in the mitral arcade. Leaflet mobility can be severely restricted, involving both anterior and posterior leaflets. These leaflets are often affected beginning at the base of the leaflet that extends towards the free edge. The membrane type of tissue most often can be peeled off the native leaflet using a combination of sharp and blunt dissection, similar to the technique of endarterectomy in a diseased vessel. The leaflet edge is often rolled, and extra care must be taken to prevent significant leaflet injury. Commissurotomy can manage commissural leaflet fusion. As the leaflet edges are derided, the thickened chords associated with a mitral arcade are approached.
Subvalvar
The significant abnormality in congenital MV stenosis is the abnormally developed papillary muscles and associated chordal structures. These structures are often fused, and removing the connective tissue layer can be complex, and sharp chordal splitting is often required, fused chordae and papillary muscle are treated by chordal division and muscle splitting, respectively, to restore proper leaflet motion (43). In young infants, this can be very challenging because the chords cannot be easily identified, risking transaction and creating of a flail segment of the leaflet (44).
Assessment of MV repair
Except intro-operative echocardiographic assessment, the effectiveness of the repair is commonly tested by injecting saline through the MV into the left ventricular cavity with a bulb syringe. The result is judged to be satisfactory when the following conditions are accomplished: (I) the closure line of the two leaflets lies parallel to the posterior portion of annulus; (II) the surface of coaptation between the two leaflets is greater than 3 mm; (III) leaflet mobility is satisfactory. An asymmetrical line of closure indicates residual prolapse or residual restricted motion of one leaflet. Small leakages have little importance as long as the three previously exposed criteria are present: they usually disappear or remain trivial once the MV is working under normal systolic pressure.
MVR
The MVR is inevitable when children have severe, difficult-to-repair MV malformations or the effects of various angioplasty are unsatisfied. If the valve is too large, it is sutured into supra-annular position with interrupted pledged sutures (45). However, this procedure may lead to left atrium pressures because of the rigid prosthetic ring loss of left atrium distensibility. The chimney technique was used to solve annulus-prosthesis mismatch without negatively affecting the opening of the leaflets. In this procedure, a custom-made composite graft with a 16-mm Carbomedics mitral prosthesis and a 20-mm Dacron graft is used and implanted in the supra-annular position (46-48). In general, mechanical valves and stented bovine jugular vein valves are preferable to bioprosthetic valves due to less calcification and better durability (49-51). Pulmonary autograft mitral valve replacement (PA-MVR), also known as ROSS II, was introduced clinically in infants with intractable CMVM. During this procedure, reinforcement of the pulmonary outer wall is required to retain a suitable valve function. Besides, reinforcement with a cylinder of expanded polytetrafluoroethylene is efficient to maintain pulmonary autograft configuration and function (52-56). However, options for MVR in neonates and infants are challenging because fixed-diameter prostheses cannot accommodate somatic growth, and their MV annulus may not match larger mechanical prostheses. Therefore, numerous valve modification and implantation techniques were used to tailor surgical strategies utilized for MVR in infants to create an opportunity to perform MVR at an earlier time and achieve reliable valve function and excellent survival. Novel annular and supra-annular MVR is a helpful strategy for accommodating a larger prosthesis by modifying the small native annulus to a larger “net-annulus” (57,58). The stented bovine jugular vein graft (Melody valve) can be expanded through a balloon catheter to achieve the best diameter (12–20 mm) (59). Melody valve modification and implantation techniques, such as stent shortening, adding a pericardial sewing cuff, intraoperative balloon expansion, and fixation of the distal stent to the inferior left ventricle wall, can result in acceptable short-term function by reducing left ventricular outflow tract obstruction and paravalvular leaks. Besides, the Melody valve can be percutaneously expanded as the child grows (60).
Outcomes of surgical strategies
MV repair
In 2016, Vida reported MV regurgitation and stenosis combined studies among 100 patients who underwent MV repair, which showed actuarial survival of 95%, 94%, and 93% at 5, 10, and 20 years, respectively; 16 patients (18%) required reintervention due to subsequent MV dysfunction (61). In the Makoto series, freedom from reoperation for patients with isolated mitral regurgitation and other congenital heart diseases at 10 years was 91.7% and 68.4%; actuarial survival was 97.0% and 85.1%, respectively (25). In a large cohort study aimed at patients who underwent MV repair for MV dysplasia, operative and follow-up mortality was 0%. Reintervention in the whole population occurred in 31% of patients (62). For simply MV regurgitation study, Shi and colleagues reported that overall freedom from moderate or greater MV regurgitation was 96.3%, 91.9%, and 83.6% at 2, 5, and 10 years, respectively (63). Jiang reported that the freedom from moderate or severe regurgitation after MV repair was 92.3% (64). For simply MV stenosis study, Cho and colleagues reported that the survival rate was 85.9%±7.6% (65). Management of SMR has a relatively good outcome, with a 20-year survival rate of 82% and freedom from reoperation rate of 88%. CMVM patients with other associated cardiac anomalies would increase the risk of poor outcomes and death, such as pulmonary stenosis and left ventricular outflow tract obstruction-related etiology (66,67). Moreover, patients diagnosed with Shone’s anomaly were risk factors for late mortality (41). A 23-year follow-up of 45 infants with Shone’s anomaly showed the rate of freedom from reoperation was about 97.6%, 89.3%, 77.1%, 72.0%, and 52.8% at 30 days, 1, 5, 10, and 15 years postoperatively. Moreover, the cumulative survival rate ranges from 97.6% to 70.3% at 30 days to 15 years postoperatively (68). In addition, the importance of residual regurgitation and stenosis during MV repair should be carefully evaluated to guide intraoperative decision-making. Results of several studies showed post-repair MV regurgitation to be a predominant predictor of death or reoperation (34,69,70). Overall, MV repair shows acceptable early mortality and reoperation rates; the outcome of MV regurgitation is slightly better than MV stenosis.
MVR
MVR is an uncommon procedure in children due to higher mortality and morbidity rates than MV repair. With the development of tailored surgery techniques and durable prostheses, MVR has been successfully performed to satisfy eligible patients’ short-term and long-term outcomes. Brancaccio et al. (71) conducted a retrospective study including 115 patients undergoing MVR and reported death or transplant-free survival was nearly 77% at 5 years and 72% at 10 years. Freedom from MV re-replacement was 90% and 72% at 5 and 10 years. A sizeable multi-institutional study showed that the early mortality (<90 days) was 11.1%, and the risk could be increased by younger age (<2 years) and concurrent different mechanical valve placement. Moreover, among those children who survived more than 90 days, the transplant-free survival was 76% at 20 years of follow-up (72). MVR in neonates and infants is much challenging. Moon et al. (58) reported that overall survival rates and freedom from redoing the MVR at 10 years were 88.9% and 57.8% among 18 infants who underwent the 16-mm TAS mechanical valves. A multicenter cohort study involving 17 infants documented that the 15-mm prosthesis can be safely performed in neonates and infants, and the median freedom from redoing MVR for outgrowth was 3.5 years (73).
Further research
CMVM in pediatric patients encompasses a broad range of pathology, varying in different anatomical abnormalities. Though the prevalence of CMVM is rare, it has a significant burden of morbidity and mortality on carriers. Surgical strategies in infants and children pose numerous challenges. Outcomes for surgical management of MV disease is depended on the patient’s age and weight, the anatomic substrate, associated cardiac anomalies, the presence or absence of pulmonary hypertension, and center expertise. Improvements in pre-operative, intraoperative, and post-operation assessment techniques benefit from providing congenital heart surgeons with accurate evaluations of MV pathology. Although MVR among infants has obtained encouraging results, a more extensive study is needed to verify the safety and effectiveness of operations.
Moreover, modification of valve design and implantation techniques is necessary to reduce complications and residual regurgitation. Future advances with a tissue-engineered prosthesis may change the landscape for valve replacement in the pediatric population. In addition, research on CMVM surgical strategies and outcomes are predominantly retrospective studies reviewing cases over a long period, and therefore recall time-effects are inevitable due to rapidly developed surgical techniques. Further prospective multicenter studies may provide updated and improved evidence and achieve better outcomes for patients.
Acknowledgments
Funding: This work was supported by the Shanghai Natural Science Foundation of Science and Technology Innovation Action Plan (Grant No. 21ZR1409900).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Swee Chye Quek and Yiu-Fai Cheung) for the series “Advances in Pediatric Cardiology” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-52/rc
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-52/coif). The series “Advances in Pediatric Cardiology” was commissioned by the editorial office without any funding or sponsorship. GH serves as the Editor-in-Chief of Pediatric Medicine. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Douedi S, Douedi H. Mitral Regurgitation. Treasure Island, FL, USA: StatPearls Publishing, 2021.
- Banerjee A, Kohl T, Silverman NH. Echocardiographic evaluation of congenital mitral valve anomalies in children. Am J Cardiol 1995;76:1284-91. [Crossref] [PubMed]
- Rosenquist GC. Congenital mitral valve disease associated with coarctation of the aorta: a spectrum that includes parachute deformity of the mitral valve. Circulation 1974;49:985-93. [Crossref] [PubMed]
- Ruckman RN, Van Praagh R. Anatomic types of congenital mitral stenosis: report of 49 autopsy cases with consideration of diagnosis and surgical implications. Am J Cardiol 1978;42:592-601. [Crossref] [PubMed]
- Prifti E, Vanini V, Bonacchi M, et al. Repair of congenital malformations of the mitral valve: early and midterm results. Ann Thorac Surg 2002;73:614-21. [Crossref] [PubMed]
- Carpentier A, Branchini B, Cour JC, et al. Congenital malformations of the mitral valve in children. Pathology and surgical treatment. J Thorac Cardiovasc Surg 1976;72:854-66.
- David TE. Perspectives on surgical treatment of mitral valve disease. Asian Cardiovasc Thorac Ann 2020;28:360-5. [Crossref] [PubMed]
- David TE. Commentary: Left ventricular function after mitral valve repair. J Thorac Cardiovasc Surg 2022;164:1499-500. [Crossref] [PubMed]
- Vida VL, Zanotto L, Carrozzini M, et al. Repair Techniques for Mitral Valve Insufficiency in Children. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2018;21:41-5. [Crossref] [PubMed]
- Cantinotti M, Giordano R, Koestenberger M, et al. Echocardiographic examination of mitral valve abnormalities in the paediatric population: current practices. Cardiol Young 2020;30:1-11. [Crossref] [PubMed]
- Arbic N, Dragulescu A, Mertens L, et al. The Use of 3D Echocardiography in Surgical Planning of the Mitral Valve in Pediatric Cardiology. J Vis Exp 2021;e62574. [Crossref] [PubMed]
- Jolley MA, Ghelani SJ, Adar A, et al. Three-Dimensional Mitral Valve Morphology and Age-Related Trends in Children and Young Adults with Structurally Normal Hearts Using Transthoracic Echocardiography. J Am Soc Echocardiogr 2017;30:561-71. [Crossref] [PubMed]
- Valverde I, Rawlins D, Austin C, et al. Three-dimensional echocardiography in the management of parachute mitral valve. Eur Heart J Cardiovasc Imaging 2012;13:446. [Crossref] [PubMed]
- Ge S, Bu L, Zhang H, et al. A real-time 3-dimensional digital Doppler method for measurement of flow rate and volume through mitral valve in children: a validation study compared with magnetic resonance imaging. J Am Soc Echocardiogr 2005;18:1-7. [Crossref] [PubMed]
- Garg P, Swift AJ, Zhong L, et al. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat Rev Cardiol 2020;17:298-312. [Crossref] [PubMed]
- Freeman WK, Schaff HV, Khandheria BK, et al. Intraoperative evaluation of mitral valve regurgitation and repair by transesophageal echocardiography: incidence and significance of systolic anterior motion. J Am Coll Cardiol 1992;20:599-609. [Crossref] [PubMed]
- Rosenfeld HM, Gentles TL, Wernovsky G, et al. Utility of intraoperative transesophageal echocardiography in the assessment of residual cardiac defects. Pediatr Cardiol 1998;19:346-51. [Crossref] [PubMed]
- Madriago EJ, Punn R, Geeter N, et al. Routine intra-operative trans-oesophageal echocardiography yields better outcomes in surgical repair of CHD. Cardiol Young 2016;26:263-8. [Crossref] [PubMed]
- Van DH, Pham NHM, Nguyen VMT, et al. Isolated Congenital Mitral Regurgitation Repair in Children: Long-term Outcomes of Artificial Rings. Ann Thorac Surg 2022;113:638-45. [Crossref] [PubMed]
- Yakub MA, Sivalingam S, Dillon J, et al. Mitral valve repair for congenital mitral valve disease: impact of the use of a biodegradable annuloplasty ring. Ann Thorac Surg 2015;99:884-90; discussion 890. [Crossref] [PubMed]
- Zaidi AH, Nathan M, Emani S, et al. Preliminary experience with porcine intestinal submucosa (CorMatrix) for valve reconstruction in congenital heart disease: histologic evaluation of explanted valves. J Thorac Cardiovasc Surg 2014;148:2216-4, 2225.e1.
- Liu LX, Zhang JF, Xu WX, et al. Mitral valve repair using an autologous pericardial strip in infants and young children. J Card Surg 2017;32:45-8. [Crossref] [PubMed]
- Maeda T, Fujiwara K, Yoshizawa K, et al. Posterior mitral leaflet extension using autologous pericardium to repair a hammock mitral valve associated with severe mitral valve regurgitation in a 4-month-old boy. Gen Thorac Cardiovasc Surg 2020;68:1203-7. [Crossref] [PubMed]
- Brizard CP, Brink J, Horton SB, et al. New engineering treatment of bovine pericardium confers outstanding resistance to calcification in mitral and pulmonary implantations in a juvenile sheep model. J Thorac Cardiovasc Surg 2014;148:3194-201. [Crossref] [PubMed]
- Ando M, Takahashi Y. Durability of Mitral Valve Repair Performed Before the Age of 5 Years. Circ J 2016;80:124-9. [Crossref] [PubMed]
- Sorajja P, Ukaigwe AC. Edge-to-edge repair: past challenge, current case selection and future advances. Ann Cardiothorac Surg 2021;10:43-9. [Crossref] [PubMed]
- Ando M, Takahashi Y. Edge-to-edge repair of common atrioventricular or tricuspid valve in patients with functionally single ventricle. Ann Thorac Surg 2007;84:1571-6; discussion 1576-7. [Crossref] [PubMed]
- Quarti A, D'alfonso A, Colaneri M, et al. Edge-to-edge technique: is it also useful in children? J Cardiovasc Med (Hagerstown) 2009;10:848-51. [Crossref] [PubMed]
- Buratto E, Ye XT, Konstantinov IE. Mitral repair in children with connective tissue disorders: On the edge, over the edge, or edge-to-edge? J Thorac Cardiovasc Surg 2017;153:404-5. [Crossref] [PubMed]
- Gorenflo M, Katus HA, Bekeredjian R. Successful MitraClipTM implantation in a 15-year-old patient with multiple prior cardiac surgeries. Cardiol Young 2013;23:620-2. [Crossref] [PubMed]
- Joffe DC, Jones TK, Reisman M, et al. Not for adults only: MitraClip use in a paediatric patient. EuroIntervention 2016;12:e1065-70. [Crossref] [PubMed]
- Perrault LP, Moskowitz AJ, Kron IL, et al. Optimal surgical management of severe ischemic mitral regurgitation: to repair or to replace? J Thorac Cardiovasc Surg 2012;143:1396-403. [Crossref] [PubMed]
- Gillinov AM, Cosgrove DM. Chordal transfer for repair of anterior leaflet prolapse. Semin Thorac Cardiovasc Surg 2004;16:169-73. [Crossref] [PubMed]
- Sivalingam S, Haranal M, Moorthy PSK, et al. Mid-Term Results Comparing the Use of Artificial Chords Versus Native Chords for Mitral Valve Repair in Children. World J Pediatr Congenit Heart Surg 2020;11:579-86. [Crossref] [PubMed]
- Minami K, Kado H, Sai S, et al. Midterm results of mitral valve repair with artificial chordae in children. J Thorac Cardiovasc Surg 2005;129:336-42. [Crossref] [PubMed]
- Boon R, Hazekamp M, Hoohenkerk G, et al. Artificial chordae for pediatric mitral and tricuspid valve repair. Eur J Cardiothorac Surg 2007;32:143-8. [Crossref] [PubMed]
- Murashita T, Hoashi T, Kagisaki K, et al. Long-term results of mitral valve repair for severe mitral regurgitation in infants: fate of artificial chordae. Ann Thorac Surg 2012;94:581-6. [Crossref] [PubMed]
- Oda S, Nakano T, Tatewaki H, et al. A 17-year experience with mitral valve repair with artificial chordae in infants and children. Eur J Cardiothorac Surg 2013;44:e40-5. [Crossref] [PubMed]
- García Fuster R, Martín E, Paredes F, et al. Artificial chordae in the setting of complex mitral valve repair: early outcomes using the folding leaflet technique. Interact Cardiovasc Thorac Surg 2014;18:586-95. [Crossref] [PubMed]
- Kluin J, Sojak V, Koolbergen DR, et al. Fifteen years' experience with the use of artificial chords for valve reconstruction in children. Eur J Cardiothorac Surg 2017;52:1155-60. [Crossref] [PubMed]
- Brown JW, Ruzmetov M, Rodefeld MD, et al. Surgical strategies and outcomes in patients with supra-annular mitral ring: a single-institution experience. Eur J Cardiothorac Surg 2010;38:556-60. [Crossref] [PubMed]
- SHONE JD. The developmental complex of "parachute mitral valve," supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol 1963;11:714-25. [Crossref] [PubMed]
- Carpentier A, Brizard C. Congenital Malformations of the Mitral Valve. In: Stark JF, de Leval MR, Tsang VT. editors. Surgery for Congenital Heart Defects. Hoboken, NJ, USA: Wiley, 2006.
- Burbano NH. Congenital Mitral Stenosis. J Cardiothorac Vasc Anesth 2020;34:2272-3. [Crossref] [PubMed]
- Brown JW, Fiore AC, Ruzmetov M, et al. Evolution of mitral valve replacement in children: a 40-year experience. Ann Thorac Surg 2012;93:626-33; discussion 633. [Crossref] [PubMed]
- González Rocafort Á, Aroca Á, Polo L, et al. Chimney technique for mitral valve replacement in children. Ann Thorac Surg 2013;96:1885-7. [Crossref] [PubMed]
- Cakir H, Kestelli M, Yurekli I, et al. About Chimney Technique for Mitral Valve Replacement. Ann Thorac Surg 2015;100:771. [Crossref] [PubMed]
- Go S, Furukawa T, Yamada K, et al. A case of supra-annular mitral valve replacement using chimney technique for severe mitral stenosis with extensive mitral annular calcification. Gen Thorac Cardiovasc Surg 2020;68:1199-202. [Crossref] [PubMed]
- van Doorn C, Yates R, Tsang V, et al. Mitral valve replacement in children: mortality, morbidity, and haemodynamic status up to medium term follow up. Heart 2000;84:636-42. [Crossref] [PubMed]
- Alexiou C, Galogavrou M, Chen Q, et al. Mitral valve replacement with mechanical prostheses in children: improved operative risk and survival. Eur J Cardiothorac Surg 2001;20:105-13. [Crossref] [PubMed]
- Choi PS, Sleeper LA, Lu M, et al. Revisiting prosthesis choice in mitral valve replacement in children: Durable alternatives to traditional bioprostheses. J Thorac Cardiovasc Surg 2020;S0022-5223(20)31281-2.
- Kanzaki T, Yamagishi M, Yashima M, et al. Seven-year outcome of pulmonary valve autograft replacement of the mitral valve in an infant. J Thorac Cardiovasc Surg 2011;141:e33-5. [Crossref] [PubMed]
- Fedevych O, Yachnik O, Mykychak Y, et al. Ross-Konno Procedure With Cylinder Mitral Valve Replacement in 49 Days Old Infant. World J Pediatr Congenit Heart Surg 2018;9:587-90. [Crossref] [PubMed]
- Jeong YH, Yun TJ. Mitral Valve Replacement with a Pulmonary Autograft in an Infant. Korean J Thorac Cardiovasc Surg 2018;51:149-52. [Crossref] [PubMed]
- Moreau de Bellaing A, Mathiron A, Lecompte Y, et al. Mitral valve replacement with a pulmonary autograft: long-term follow-up in an infant. Interact Cardiovasc Thorac Surg 2019;28:828-9. [Crossref] [PubMed]
- Zou MH, Ma L, Yang SC, et al. The early results of pulmonary autograft mitral valve replacement (Ross II) in infants. Zhonghua Wai Ke Za Zhi 2020;58:793-7. [Crossref] [PubMed]
- Carroll ND, Beers KM, Maldonado EM, et al. Novel Annular and Subvalvular Enlargement in Congenital Mitral Valve Replacement. Ann Thorac Surg 2016;102:e277-9. [Crossref] [PubMed]
- Moon J, Hoashi T, Kagisaki K, et al. Clinical outcomes of mitral valve replacement with the 16-mm ATS advanced performance valve in neonates and infants. Ann Thorac Surg 2015;99:653-9. [Crossref] [PubMed]
- Frigiola A, Pluchinotta FR, Saracino A, et al. Surgical mitral valve replacement with the Melody valve in infants and children: the Italian experience. EuroIntervention 2017;12:2104-9. [Crossref] [PubMed]
- Quiñonez LG, Breitbart R, Tworetsky W, et al. Stented bovine jugular vein graft (Melody valve) for surgical mitral valve replacement in infants and children. J Thorac Cardiovasc Surg 2014;148:1443-9. [Crossref] [PubMed]
- Vida VL, Carrozzini M, Padalino M, et al. Surgical Treatment of Congenital Mitral Valve Dysplasia. J Card Surg 2016;31:352-6. [Crossref] [PubMed]
- Brancaccio G, Chinali M, Trezzi M, et al. Outcome for Conservative Surgery for the Correction of Severe Mitral Valve Regurgitation in Children: A Single-Center Experience. Pediatr Cardiol 2019;40:1663-9. [Crossref] [PubMed]
- Shi Y, Xu H, Yan J, et al. The Mid-term Results of Mitral Valve Repair for Isolated Mitral Regurgitation in Infancy and Childhood. Pediatr Cardiol 2017;38:1592-7. [Crossref] [PubMed]
- Jiang Z, Mei J, Ding F, et al. The early and mid-term results of mitral valve repair for mitral regurgitation in children. Surg Today 2014;44:2086-91. [Crossref] [PubMed]
- Cho S, Kim WH, Kwak JG, et al. Surgical results of mitral valve repair for congenital mitral valve stenosis in paediatric patients. Interact Cardiovasc Thorac Surg 2017;25:877-82. [Crossref] [PubMed]
- Baghaei R, Tabib A, Jalili F, et al. Early and Mid-Term Outcome of Pediatric Congenital Mitral Valve Surgery. Res Cardiovasc Med 2015;4:e28724. [Crossref] [PubMed]
- Kalfa D, Vergnat M, Ly M, et al. A standardized repair-oriented strategy for mitral insufficiency in infants and children: midterm functional outcomes and predictors of adverse events. J Thorac Cardiovasc Surg 2014;148:1459-66. [Crossref] [PubMed]
- Delmo Walter EM, Komoda T, Siniawski H, et al. Long-term surgical outcome of mitral valve repair in infants and children with Shone's anomaly. Eur J Cardiothorac Surg 2013;43:473-81; discussion 481-2. [Crossref] [PubMed]
- Alghamdi AA, Yanagawa B, Singh SK, et al. Balancing stenosis and regurgitation during mitral valve surgery in pediatric patients. Ann Thorac Surg 2011;92:680-4. [Crossref] [PubMed]
- Yakub MA, Krishna Moorthy PS, Sivalingam S, et al. Contemporary long-term outcomes of an aggressive approach to mitral valve repair in children: is it effective and durable for both congenital and acquired mitral valve lesions? Eur J Cardiothorac Surg 2016;49:553-60; discussion 560. [Crossref] [PubMed]
- Brancaccio G, Trezzi M, Chinali M, et al. Predictors of survival in paediatric mitral valve replacement. Eur J Cardiothorac Surg 2021;60:361-6. [Crossref] [PubMed]
- Ibezim C, Sarvestani AL, Knight JH, et al. Outcomes of Mechanical Mitral Valve Replacement in Children. Ann Thorac Surg 2019;107:143-50. [Crossref] [PubMed]
- IJsselhof RJ, Slieker MG, Gauvreau K, et al. Mechanical Mitral Valve Replacement: A Multicenter Study of Outcomes With Use of 15- to 17-mm Prostheses. Ann Thorac Surg 2020;110:2062-9. [Crossref] [PubMed]
Cite this article as: Chen W, Li M, Sheng W, Jia B, Huang G. Congenital mitral valve defects in pediatric population: a narrative review of surgical repair. Pediatr Med 2023;6:6.

