
International network for evaluating outcomes of neonates: outputs and future directions
Background information
Ten neonatal networks from 11 countries—Australia, New Zealand, Canada, Finland, Israel, Japan, Spain, Sweden, Switzerland, the Tuscany region of Italy, and the UK—came together in 2012 to form the International Network for Evaluating Outcomes of Neonates (iNeo): an international collaboration of population-representative, national neonatal datasets. The result is a powerful platform for epidemiological, outcomes-based, and applied health services and policy research. Our underlying goal in building this collaboration was to improve patient-oriented outcomes for neonates born very preterm (VPT, born before 32 weeks’ gestational age) and extremely preterm (EPT, born before 28 weeks’ gestation) around the world.
As outlined by others in this article series, most of the networks involved in iNeo have well-established platforms for their own internal evaluation, benchmarking, and quality improvement activities. They also participate in many benchmarking activities both nationally and internationally. Since inception, iNeo has produced several high-quality outputs in the domains of outcomes evaluation, evaluation of care and practice similarities and differences between the networks, epidemiological studies, and health services evaluation. In this article we will review the salient features of these outputs and identify opportunities for the next phase in this influential international collaboration.
Overarching network aims
As detailed in the following sections, the iNeo collaboration has made steady progress towards achieving many of its initial aims (1): these aims were to compare neonatal outcomes and health service organization for VPT neonates at the national level; to identify differences in site-level physical, human, and environmental characteristics, as well as care practices, that underlie the outcome variations; to identify clinical and organizational practice improvements relevant to each network; to implement and continually evaluate the impact of data-informed and evidence-linked clinical and organizational practice changes in neonatal intensive care units (NICUs) within participating networks; and to train and mentor junior researchers in the conduct of neonatal-perinatal health services research.
Network structure and organization
The iNeo collaboration was established in 2013 with funds from the Canadian Institutes of Health Research’s Institute of Human Development, Child and Youth Health (2). Its purpose is to collect phenotypic information on newborns admitted to neonatal units in the participating countries, along with some maternal details, at the time of birth. As this information is already collected in local datasets, the purpose of iNeo is to first harmonize and then collectively assemble a larger pool of individual patient data from the original, population-based networks or datasets. Harmonization efforts like these have even stimulated interest and opportunities in country like China, which recently reported their first cohort data from 68 neonatal units (3-5). The underlying principles of the iNeo collaboration were to study variations in the outcomes, characteristics, practices, and cultures of the member sites; evaluate the impact of such variations on neonatal outcomes; and identify and learn from different models of health service delivery (incorporating medical and non-medical variations). The results have been published and advertised by respective network to their constituents to implement data-linked and evidence-based practice changes where they are applicable, feasible, and sensible for their own environments.
The organization, their structure, underlying population-bases, and linkages with extended datasets of the individual networks have been well characterized in a review article demonstrating the evolution of iNeo (1).
Current collaboration
The following neonatal networks are currently participating in the iNeo collaboration: Australian and New Zealand Neonatal Network (ANZNN); Canadian Neonatal Network (CNN); Finnish Medical Birth Register (FinMBR); Israel Neonatal Network (INN); Neonatal Research Network Japan (NRNJ); Spanish Neonatal Network (SEN1500); Swedish Neonatal Quality Register (SNQ); Swiss Neonatal Network (SNN); Tuscany region of Italy (TuscanNN); and UK Neonatal Collaborative (UKNC).
Dataset development and modification
After a detailed review of all data items collected by the participating networks, a minimum dataset was conceived in July, 2012. Variations in definitions were harmonized for inclusion in the minimum database and were mapped to the International Classification of Diseases and Related Health Problems (ICD-10) (6) and Systematized Nomenclature of Medicine (SNOMED) (7) dictionaries. The minimal dataset has been modified with the addition of some variables. The process of data collection and transfer has been streamlined and described previously (1,8). The system is organized in such a way that data are received at coordinating center from the respective countries as and when they become available. The numbers of neonates currently available from each country and certain baseline characteristics of each organization are reported in Table 1. Ethics approval is from local network regulatory authorities and the Research Ethics Board at Mount Sinai Hospital, Toronto, which is the coordinating center for the iNeo collaboration.
Table 1
| Items | ANZNN | CNN | FinMBR | INN | NRNJ | SEN1500 | SNQ | SNN | TuscanNN | UKNC |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of live births from national data (year of report) | Australia: 305,832 (2019); New Zealand: 57,753 (2020) |
372,038 (2019) | 46,463 (2020) |
182,016 (2019) | 840,832 (2020) | 339,206 (2020) | 113,017 (2020) | 85,914 (2020) | 23,462 (2019) | 687,179 (2019) |
| Number of neonatal units contributing data to the network in 2017 | 29 | 30 | 31 | 28 | 219 | 75 | 38 | 10 | 20 | 129 |
| Network contribution | National | National | National | National | National-partial | National-partial | National | National | Regional | National-partial |
| National recommendations for transfer of pregnant women | Australia: <33 weeks; New Zealand: <34 weeks |
<32 weeks | <30-32 weeks | No | No | <32 weeks | <26 weeks | <32 weeks | <32 weeks | <28 weeks |
| Designated neonatal transport teams | Yes | Yes | No | No | No | Yes, in some regions | Yes | Yes | Yes | Yes |
| Average total number of neonates for whom data are contributed per year | 3,896 | 3,658 | 479 | 1,428 | 4,813 | 2,838 | 1,249 | 871 | 339 | 9,598 |
| Average total number of neonates per year in data who are born at 24–28 weeks’ gestation | 1,486 | 1,513 | 171 | 582 | 1,990 | 1,122 | 384 | 307 | 102 | 2,951 |
| GA in weeks, mean (SD) | 26.4 (1.4) | 26.3 (1.4) | 26.4 (1.4) | 26.4 (1.4) | 26.3 (1.4) | 26.4 (1.3) | 26.4 (1.4) | 26.5 (1.3) | 26.3 (1.4) | 26.4 (1.4) |
| BW in grams, mean (SD) | 940 (250.0) | 940 (250.0) | 933 (254.0) | 906 (221.0) | 862 (231.0) | 913 (224.0) | 930 (252.0) | 896 (235.0) | 884 (247.0) | 921 (229.0) |
| Male sex, No. (%) | 8,872 (54.3) | 9,893 (54.6) | 1,097 (53.5) | 3,842 (55.0) | 11,780 (53.8) | 6,643 (53.8) | 2,325 (55.1) | 1,813 (53.7) | 493 (53.7) | 15,563 (54.0) |
ANZNN, Australian and New Zealand Neonatal Network; CNN, Canadian Neonatal Network; FinMBR, Finnish Medical Birth Register; INN, Israel Neonatal Network; NRNJ, Neonatal Research Network Japan; SEN1500, Spanish Neonatal Network; SNQ, Swedish Neonatal Quality Register; SNN, Swiss Neonatal Network; TuscanNN, Tuscany Neonatal Network; UKNC, UK Neonatal Collaborative; Last 5 rows present data for 2007–2017 for ANZNN, NRNJ, SEN1500, SNQ, and SNN; for 2007-2018 for CNN, FinMBR, and INN; for 2009–2017 for TuscanNN; and for 2008–2017 for UKNC. BW, birth weight; GA, gestational age; SD, standard deviation.
Network outputs/achievements
The main purpose of this review is to present a succinct report of iNeo outputs and identify opportunities for the next phase of this collaboration. Key iNeo outputs are as follows:
Overarching project documents with report of health services organization
After the iNeo collaboration was formed, its first act was to develop and publish a protocol. We developed a detailed plan following our mission and aims, which was first published along with the guidance document for data transfer and the data elements (8). We developed rules of engagement and conduct for different projects. There were several concerns in the initial stage of the project with regards to differences in health care services provision and health care services organization related to pregnancy and childbirth at national and even local level. The first order of the collaboration was to conduct a survey of the network directors or contact persons and collect information from the existing national documents or databases as to how neonatal-perinatal services for EPT neonates were organized within the individual countries (9). In that project, we identified that all participating countries have nationally funded maternal neonatal health care services and more than 90% of women receive prenatal care. Variations are identified and reported in Table 1; however, several similarities were also identified. In 2017, after an annual meeting of the collaboration directors, it was decided that the database should be expanded to collect additional details. These included data on daily processes of care and the interventions EPT neonates receive in NICU. The database was subsequently complemented with several additional variables, which were also published to ensure transparency (1).
Evaluating outcomes
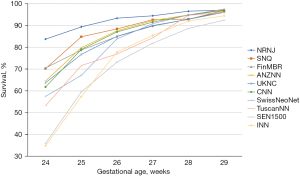
One of the major purposes of our international collaboration was to evaluate the reasons for any variations in neonatal outcomes identified. In one of our initial papers (10), we analyzed common neonatal outcomes of preterm neonates born at 24 weeks to 32 weeks’ gestation and admitted to neonatal units between the years 2007 and 2010. In that study of >58,000 neonates, we identified that the composite outcome of mortality, bronchopulmonary dysplasia (BPD: defined as need for oxygen support at 36 weeks’ post-menstrual age), treated retinopathy of prematurity, and severe neurological injury varied from 26% to 42% among countries. Overall mortality in the cohort was 10%; however, it varied from 5% in Japan to 17% in Spain. The standardized ratio for the composite outcome are shown in Figure 1. We speculated that the differences could be due to variation in population coverage, data collection, and/or case definition; and we called for further harmonization. Once further data were available, we aimed to identify outcome trends in participating countries over the time (11). Due to significant variations in management of neonates of 22 and 23 weeks’ gestational age, the collaboration has focused on neonates of >24 weeks’ gestation as these neonates were universally provided neonatal care in all countries during study period. Lui et al. (11) evaluated >154,000 preterm neonates admitted to 529 neonatal units across iNeo countries between 2007 and 2015 (Table 2). We identified increases and decreases over the study years when the composite outcome included or excluded BPD. We also observed that this trend was consistently reduced in the later years of the study in Canada, which coincided with national quality improvement efforts. In most countries, mortality for preterm neonates was reduced over years; however, Helenius et al. undertook a project to understand the distribution of mortality rates in relation to gestational age (12). We studied neonates born between 24 weeks’ and 29 weeks’ gestation and admitted between 2007 and 2013. The survival rate increased as gestational age increased; however, differences between the countries remained relatively similar at all gestational ages (Figure 2). We identified that standardized ratios for survival were highest for Japan and lowest for Spain, and the overall survival ranged from 78% to 93% between networks. This finding has prompted investigations at local unit and within-country levels. Moreover, prompted by the identification of between-network variation in the outcome of bronchopulmonary dysplasia, Hines et al. (13) undertook a systematic review of different definitions for BPD used by various investigators. We reviewed publications between 2010 and 2015 that reported on BPD as an outcome and compared the different definitions used. We noted that rates of BPD ranged from 6% to 57%, and the rate reported was entirely dependent on the definition chosen for the study. We also identified that BPD had a moderate predictive value with regards to long term pulmonary and neurosensory outcomes due to variations in the definitions. A call was made to develop a comprehensive and evidence-based definition of BPD for the purposes of benchmarking.
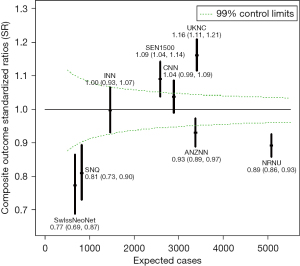
Table 2
| Network | Outcome | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | P value (Trend) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANZNN | Including BPD | 54.6 | 50.9 | 52.9 | 56.6 | 57.8 | 57.8 | 61.5 | 63.2 | 63.9 | 64.8 | <0.01* |
| Excluding BPD | 28.9 | 26.6 | 26.2 | 26.6 | 25.7 | 23.4 | 24.8 | 27.2 | 25.2 | 24.5 | 0.02** | |
| CNN | Including BPD | 69.5 | 66.1 | 66.1 | 59.7 | 60.6 | 58.1 | 55.9 | 56.7 | 54.7 | 53.6 | <0.01** |
| Excluding BPD | 40.3 | 40.7 | 39.7 | 28.8 | 29.5 | 28.8 | 28.7 | 29 | 26.8 | 29.7 | <0.01** | |
| FinMBR | Including BPD | 57.9 | 57.2 | 57.0 | 44.7 | 53.5 | 44.2 | 46.9 | 44.9 | 53.2 | 47.1 | 0.01** |
| Excluding BPD | 32.8 | 31.0 | 37.8 | 26.7 | 29.2 | 22.4 | 26.1 | 28.0 | 24.3 | 23.3 | <0.01** | |
| INN | Including BPD | 56.8 | 55.0 | 57.0 | 56.2 | 53.7 | 58.3 | 55.1 | 51.2 | 53.4 | 56.4 | 0.26 |
| Excluding BPD | 46.7 | 44.2 | 46.9 | 46.5 | 41.0 | 47.8 | 43.3 | 41.0 | 38.4 | 40.3 | <0.01** | |
| NRNJ | Including BPD | 55.9 | 52.9 | 52.9 | 58.1 | 57.5 | 54.6 | 57.5 | 61.1 | 60.6 | 59.7 | <0.01* |
| Excluding BPD | 43.0 | 38.1 | 36.3 | 37.9 | 35.4 | 34.7 | 32.7 | 33.5 | 35.1 | 35.5 | <0.01** | |
| SEN1500 | Including BPD | 57.5 | 54.8 | 59.1 | 60.4 | 58.7 | 58 | 58.6 | 62.2 | 60.2 | 53.5 | 0.63 |
| Excluding BPD | 47.3 | 44.3 | 47.2 | 48.6 | 45.9 | 45.6 | 45.9 | 50.8 | 50.3 | 44.0 | 0.44 | |
| SNQ | Including BPD | 51.8 | 51.0 | 50.3 | 56.8 | 53.7 | 59.5 | 52.8 | 59.8 | 63.1 | 56.1 | <0.01* |
| Excluding BPD | 31.5 | 30.3 | 32.0 | 37.3 | 32.1 | 31.0 | 29.7 | 30.7 | 34.5 | 31.3 | 0.93 | |
| SwissNeoNet | Including BPD | 37.3 | 38.6 | 41.3 | 44.2 | 34.9 | 40.7 | 42.1 | 43.5 | 42.2 | 38.3 | 0.45 |
| Excluding BPD | 28.2 | 25.6 | 27.1 | 29.4 | 23.4 | 21.3 | 24.4 | 24.4 | 24.8 | 21.7 | 0.04** | |
| TuscanNN | Including BPD | NA | NA | 60.2 | 58.3 | 58.3 | 61.5 | 59.2 | 55.8 | 40.2 | 51.7 | 0.01** |
| Excluding BPD | NA | NA | 50.9 | 50.5 | 47.2 | 47.9 | 48.5 | 50.0 | 36.3 | 46.1 | 0.12 | |
| UKNC | Including BPD | NA | 59.7 | 59.8 | 63.1 | 64.3 | 67.6 | 68.1 | 68.9 | 66.3 | 67.4 | <0.01* |
| Excluding BPD | NA | 31.0 | 29.1 | 31.1 | 30.7 | 32.2 | 31.9 | 32.2 | 30.9 | 31.1 | 0.21 |
*, P value for trend increasing. **, P value for trend decreasing. ANZNN, Australia and New Zealand Neonatal Network; BPD, bronchopulmonary dysplasia; CNN, Canadian Neonatal Network; FinMBR, Finnish Medical Birth Register; INN, Israel Neonatal Network; NA, not available; NRNJ, Neonatal Research Network of Japan; SEN1500, Spanish Neonatal Network; SNQ, Swedish National Quality Register; SwissNeoNet, Swiss Neonatal Network; TuscanNN, Tuscany Neonatal Network, Tuscany, Italy; UKNC, United Kingdom Neonatal Collaborative. Adapted with permission from Lui et al. Semin Fetal Neonatal Med 2021;26(1):101196. doi: 10.1016/j.siny.2021.101196.
Another outcome with significant variability between countries was retinopathy of prematurity (ROP). Darlow et al. (14) studied variations in ROP rates in neonates born at 24 weeks to 28 weeks’ gestation. In a study of >48,000 infants, rates of any retinopathy varied from 25% to 91% among countries in iNeo, and rates of treatment for ROP varied from 4% to 30% (Table 3).
Table 3
| Network | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | P value for Trend |
|---|---|---|---|---|---|---|---|---|
| ANZNN | 71 (8.2) | 72 (8.3) | 58 (7.4) | 46 (6.1) | 64 (7.8) | 57 (6.9) | 56 (6.6) | 0.13 |
| CNN | 88 (14.8) | 86 (13.2) | 70 (10.8) | 91 (11.6) | 71 (9.8) | 67 (8.8) | 64 (8.9) | <0.01 |
| INN | 38 (13.9) | 35 (13.0) | 33 (11.4) | 24 (7.8) | 31 (10.7) | 24 (9.3) | 25 (8.3) | 0.01 |
| NRNJ | 431 (36.4) | 311 (26.2) | 381 (30.5) | 424 (30.7) | 402 (29.5) | 439 (31.4) | 352 (28.0) | 0.03 |
| SNQ | 26 (12.8) | 21 (11.3) | 23 (11.1) | 30 (13.9) | 25 (13.5) | 18 (7.9) | 17 (6.6) | 0.02 |
| SwissNeoNet | 5 (4.0) | 7 (4.7) | 6 (4.2) | 12 (7.3) | 5 (2.8) | 6 (3.1) | 6 (3.9) | 0.49 |
| SEN1500 | 65 (14.7) | 54 (11.5) | 64 (12.5) | 77 (15.8) | 65 (13.3) | 66 (12.4) | 65 (13.2) | 0.83 |
| UKNC | NA | 59 (5.2) | 61 (5.5) | 138 (9.2) | 148 (8.8) | 142 (8.3) | 195 (11.9) | <0.01 |
| FinMBR | 11 (12.1) | 8 (7.6) | 19 (20.7) | 8 (9.1) | 14 (12.3) | 11 (11.5) | 7 (9.1) | 0.70 |
| TuscanNN | NA | NA | 8 (13.3) | 4 (8.2) | 5 (10.2) | 4 (7.7) | 6 (10.3) | 0.59 |
| Total | 735 (19.4) | 653 (13.0) | 723 (14.2) | 854 (14.9) | 830 (14.1) | 834 (13.7) | 793 (13.7) | <0.01 |
ANZNN, Australia and New Zealand Neonatal Network; CNN, Canadian Neonatal Network; FinMBR, Finnish Medical Birth Register; INN, Israel Neonatal Network; NA, data not available for this year; NRNJ, Neonatal Research Network of Japan; SEN1500, Spanish Neonatal Network; SNQ, Swedish National Quality Register; SwissNeoNet, Swiss Neonatal Network; TuscanNN, Tuscany Neonatal Network, Tuscany, Italy; UKNC, United Kingdom Neonatal Collaborative. Adapted with permission from Darlow et al. Br J Ophthalmol 2017;101:1399-1404. doi: 10.1136/bjophthalmol-2016-310041.
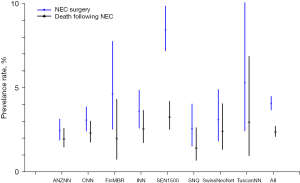
Another commonly reported outcome of EPT neonates is necrotizing enterocolitis. Adams et al. (15) used a survey-linked cohort design to study the rates of surgical necrotizing enterocolitis and practices for its prevention in 9,792 infants admitted to 8 neonatal networks (Figure 3). The standardized ratio for surgical necrotizing enterocolitis was lower for Australia-New Zealand and higher for Spain compared to overall network results. In the survey of the units participating in this study, it was noted that the provision of probiotics varied from 0% to 100% among participating units, whereas feeding initiation and advancement rates were similar.
Variations in population characteristics were identified as one possible explanation for outcome differences between countries. Maternal diabetes is associated with a 2- to 3-fold increase in the rate of very preterm birth, so we explored its relationship with the outcomes of neonates born between 24 weeks’ and 31 weeks’ gestation in iNeo countries. Persson et al. (16) compared the outcomes of 3,280 neonates born to mothers with diabetes to those of 73,080 neonates born to mothers without diabetes. We identified that gestational age and birth weight were higher in neonates born to mothers with diabetes. We also noted that mortality and composite adverse outcome rates were lower in neonates born to mothers with diabetes in unadjusted analyses; however, after adjusting for confounders, there were no significant differences in in-hospital mortality or the composite outcome between the two groups of neonates. Another maternal characteristic that has been associated with the outcomes of preterm neonates is maternal hypertension. The incidence of maternal hypertension is on the rise. Gemmell et al. (17) used the iNeo database to identify that the rate of maternal hypertension among very preterm infants varied from 11% to 16%. When the outcomes of more than 27,000 neonates born between 24 weeks’ and 29 weeks’ gestational age and admitted between 2007 and 2010 were evaluated, maternal hypertension was associated with lower odds of mortality, severe brain injury, and treated retinopathy of prematurity: but with higher odds of BPD. The authors also identified that the diagnosis of maternal hypertension varied across countries and highlighted a need for standardized diagnostic criteria. Moreover, the paradoxical impact of maternal hypertension on neonatal outcomes emphasized the importance of studying all pregnancies rather than only studying neonates born to these mothers, as there is potential for missing an intrauterine pregnancy loss.
The large international database maintained by iNeo has also allowed us to explore the possibility of understanding rare exposures. We compared the outcomes of 6,079 triplets born between 24 weeks’ and 32 weeks’ gestation to those of a matched cohort of 18,232 singleton infants (18). We identified no difference in the composite outcome of mortality, severe neurological injury, treated retinopathy of prematurity, and BPD (Table 4), and the results were robust when they were evaluated only for triplets born at 24 weeks to 28 weeks’ gestation. Norman et al. (19) compared the outcomes of 609 infants with severe congenital heart disease to those of 76,371 neonates without diagnosis of congenital heart disease and found that in-hospital mortality was significantly higher in those with CHD, with an odds ratio of 2.3 [95% confidence interval (CI): 1.61–2.37]. The large database allowed us to compare the outcomes of different types of severe congenital heart diseases. We noted that mortality was higher with all types of congenital heart disease; however, the highest odds ratios were associated with congenital heart disease causing congestive heart failure. We also identified that rates of CHD and neonatal outcomes differed significantly between countries.
Table 4
| Outcomes | Triplet neonates, N=6,079 | Singleton neonates, N=18,232 | Unadjusted odds ratio (95% CI) | Adjusted odds ratio† (95% CI) |
Adjusted odds ratio‡ (95% CI) |
|---|---|---|---|---|---|
| Composite outcome§, n (%) | 1305 (23.4) | 3,941 (24.0) | 0.97 (0.89, 1.06) | 0.91 (0.83, 1.01) | 1.00 (0.90, 1.11) |
| Mortality before discharge, n (%) | 360 (5.9) | 1,138 (6.2) | 0.95 (0.81, 1.10) | 0.83 (0.70, 0.98) | 1.08 (0.90, 1.30) |
| Severe neurological injury, n (%) | 343 (6.2) | 1,094 (6.7) | 0.92 (0.80, 1.07) | 0.91 (0.78, 1.06) | 1.12 (0.94, 1.33) |
| Treated retinopathy, n (%) | 181 (3.0) | 522 (2.9) | 1.04 (0.85, 1.28) | 0.99 (0.80, 1.22) | 1.05 (0.83, 1.32) |
| Bronchopulmonary dysplasia, n (%) | 717 (12.7) | 2,130 (12.6) | 1.01 (0.90, 1.13) | 0.97 (0.86, 1.09) | 0.94 (0.83, 1.07) |
†, Adjusted for maternal hypertension and birth weight z-score. ‡, Adjusted for maternal hypertension, cesarean birth, antenatal steroid administration, and birth weight z-score. §, Composite outcome was defined as mortality or severe neurological injury or treated retinopathy or bronchopulmonary dysplasia. CI, confidence interval; N, number in group; n, number in category. Adapted with permission from Shah PS et al. Pediatrics 2018;142(6):e20181938.
One of the major difficulties in evaluating and benchmarking outcomes between centres or between countries is the variability in severity of illness. It is argued that severity of illness varies between the units or countries and complicates outcomes comparisons unless it is adjusted for. Since no uniform severity of illness criteria are used by iNeo countries, we evaluated the universally recorded Apgar score at 5 minutes after birth as a surrogate marker of severity of illness to understand its relationship with neonatal mortality and severe neurological injury (Shah et al. 2021; submitted). In a study of 92,412 neonates, we identified that mortality decreased as 5-minute Apgar score increased from 0 to 10. Moreover, lower Apgar scores were associated with higher odds of severe neurological injury, but this relationship was not linear across the spectrum of Apgar scores.
Understanding outcome variations by evaluating practice variations using a survey and survey-linked cohort study design
After identifying outcome variations between the countries, the participants of the iNeo collaboration were determined to understand the reasons behind the variations. One of the basic dimensions in assessing system-level change in outcomes within any network is to identify improvements in outcomes over time and reduce unexplained variation in outcomes between similar constituents within the network. Lui et al. (20) evaluated outcome variations within units in each network over time in a study of 110,000 infants born at 23 weeks to 28 weeks’ gestation and admitted to 569 NICUs in 10 countries between 2007 and 2016 (Table 2). The inter-center variability in outcomes over years within individual countries increased in Australia-New Zealand, Spain, and Switzerland. Such analyses provide information crucial for identifying variations in unit-level practices as well as system capacity.
These variations were assessed in a systematic fashion after asking each unit to complete a detailed survey of physical factors, human resources, system-level factors, management practices, and availability of resources. The pre-piloted questionnaire was designed to obtain details relating to the situation in the unit in the year 2015. Shahroor et al. (21) evaluated health care personnel variations in responding units and identified that, of 325 units, 43% had team-based care models of practice and 59% (27–100% variation between countries) had in-house presence of neonatologists 24 hours per day. Regarding nursing presence, a 1:1 ratio of nursing personnel to unstable and complex care need patients was available in 52% of the units, whereas a 1:2 ratio of nursing personnel to neonates requiring multisystem support was available in 59% of the units. Other types of personnel were available in various proportions of the units, as follows, with marked variability even within countries: respiratory therapist (15%), pharmacist (40%), dietitian (34%), social worker (81%), lactation consultant (45%), parent buddy (6%).
Parents have been identified as an integral part of the care provider team for EPT neonates. Lehtonen et al. (22) evaluated facilities supporting parental presence in the infant’s room 24 hours per day in participating neonatal units. Of the 331 units that responded to a survey, only 13% had facilities accommodating infant-parent rooms in their NICUs. When patient-level data were linked for 159 units in 7 networks, we identified that 28% of the cohort was cared for in the units with infant-parent rooms. Infant outcomes are reported in Table 5. These findings highlighted the importance of family-centered care for EPT neonates, for whom the length of stay in the NICU is dependent on unit practices, discharge support, and community services organization. Seaton et al. (23) reviewed data from 28,204 neonates born at 24 weeks to 28 weeks’ gestation to understand between-country variations in length of stay for surviving neonates. Observed median length of stay was the longest in Japan (21 days longer) and was shortest in Finland (5 days shorter) than the reference country, Sweden. The factors associated with longer length of stay were country of birth, lower gestational age, multiplicity, and male sex. It was possible that differences in mortality may partially explain the longer length of stay in Japan; however, other factors could also play a role.
Table 5
| Outcomes | Unadjusted OR (95% CI) | Adjusted OR† (95% CI) | Adjusted OR‡ (95% CI) |
|---|---|---|---|
| Composite of mortality or any morbidity | 0.95 (0.84, 1.08) | 0.77 (0.65, 0.90) | 0.76 (0.64, 0.89) |
| Mortality | 0.85 (0.70, 1.02) | 0.81 (0.64, 1.02) | 0.79 (0.62, 1.00) |
| Sepsis | 0.84 (0.71, 1.00) | 0.80 (0.66, 0.98) | 0.80 (0.66, 0.97) |
| Bronchopulmonary dysplasia | 1.10 (0.95, 1.27) | 0.72 (0.61, 0.86) | 0.72 (0.61, 0.86) |
| Intraventricular hemorrhage/periventricular leukomalacia | 1.14 (0.95, 1.37) | 1.09 (0.88, 1.35) | 1.08 (0.87, 1.34) |
| Retinopathy of prematurity treatment | 0.81 (0.66, 0.99) | 0.91 (0.71, 1.16) | 0.90 (0.70, 1.15) |
| Length of stay, days | −7.5 (−10.7, −4.4) | −4.4 (−7.8, −1.1)§ | −3.4 (−4.7, −3.1)§ |
†, adjusted for gestational age, birth weight z-score, multiple birth, sex, country. ‡, Adjusted for gestational age, birth weight z-score, multiple birth, sex, country, and center volume. §, coefficient (95% CI) from general linear regression. CI, confidence interval; OR, odds ratio. Adapted with permission from Lehtonen L et al. J Pediatr 2020;226:112-7.
Several clinical practices were evaluated to identify variations between units in the management of EPT neonates. Beltempo et al. (24) evaluated practice variations in the respiratory management of EPT infants born at <29 weeks’ gestation. In a survey of 321 units, it was identified that a neonate of 23 weeks or 24 weeks’ gestation with increasing respiratory distress on continuous positive airway pressure support will be managed by most units with intubation and mechanical ventilation. However, for a neonate at 25 weeks to 26 weeks’ gestation in a similar situation, the management strategies varied significantly between the units within each network. For infants of 27 weeks and 28 weeks’ gestation, there was even more variation, with certain units providing mechanical ventilation, continuing continuous positive airway pressure support, intubation-surfactant administration-extubation, and less invasive surfactant administration. Darlow et al. (25) evaluated survey responses from 329 units about saturation target limits in the NICU. They identified that most neonatal units recently made changes to the upper and lower saturation target limits, which were now higher than previous limits. This change was reported by units in 8 out of 10 networks. They also identified that very few neonatal units set an upper target limit of >95% or a lower target limit of <85%. The concern with the changes in the oxygen saturation target was with regard to the rate of ROP. They also noted variations in criteria for retinopathy screening between neonatal units within networks, except for in Sweden, where all units followed a single guideline. Such variations could explain differences in the incidences of therapy for retinopathy between units within the network.
Isayama et al. (2021; submitted) recently evaluated whether treating pre-symptomatic patent ductus arteriosus (PDA) based on early routine echocardiography was associated with infant outcomes in a survey-linked retrospective cohort study of infants born at <29 weeks’ gestation. There was wide variation among units within the networks regarding the proportions treating asymptomatic PDA (7–86%). Of the 246 units that responded to the survey, 126 units treated pre-symptomatic PDA. The primary outcome of early death or severe neurological injury was not significantly different between neonates in the units treating vs. not treating pre-symptomatic PDA, with an adjusted odds ratio of 1.00 (95% CI: 0.85–1.18). The practice of treating pre-symptomatic PDA was associated with an increase in retinopathy of prematurity.
Helenius et al. (26) evaluated survey responses from units regarding approaches to redirection of care—especially with reference to intracranial hemorrhage, which is a complication that is not uncommon but that shows wide variations. They identified that certain units had lower rates of survivors with intracranial hemorrhage, which corresponded to higher rates of offering of redirection of care.
These variations in physical, human, and environmental unit practices and care philosophies have given us some insights into the causes of outcome variations. However, in a context where neonatal intensive care is constantly evolving (27), we need to identify additional mechanisms for collecting information on the changes and their associated effects on neonatal outcomes (5).
Studies utilizing an epidemiological underpinning
A large data set allowed us to evaluate certain controversies in the neonatal-perinatal field regarding exposure assessment and outcomes evaluation. There is an ongoing debate with regards to classification of a fetus or neonate prior to birth or at the time of birth as small for gestational age or appropriate for gestational age. Martin et al. (28) evaluated country-specific birth weight references, common birth weight references, country-specific estimated fetal weight references, and common estimated fetal weight references to classify neonates in our international cohort, and then compared their neonatal outcomes. We noted that an association of being small for gestational age with the composite outcome was similar irrespective of the classification used. We noted that small-for-gestational-age neonates had higher odds of mortality and morbidity and, although the number of infants classified as small for gestational age differed based on the references used, the risk of the composite outcome was comparable between references. Koller-Smith et al. (29) evaluated this concept further by creating two cohorts from three countries in iNeo network: very low gestational age and very low birth weight. The very low birth weight cohort had a higher number of small for gestational age infants (20% vs. 9%) and was also associated with higher rates of a composite adverse outcome compared to the very low gestational age cohort. However, the predictive powers of two models based on very low gestational age or very low birth weight for mortality and a composite outcome were similar, with areas under the curve of between 0.81 and 0.85. This allowed us to conclude that either population base is suitable for international benchmarking.
Gagliardi et al. (30) studied the “male disadvantage” that has been reported for neonatal-perinatal outcomes of EPT neonates using a large twin-pair cohort dataset from iNeo. Of the 20,924 twins in the network, approximately one third were from male-male pairs, one third were from female-female pairs, and another one third were sex discordant. The females with a male co-twin had lower odds of mortality, severe neurological injury, and a composite outcome compared to female-female pairs. Males with a female co-twin also had lower odds of mortality. Males in male-male pairs had the highest odds of BPD and composite outcomes. We concluded that sex disparity in neonatal outcomes exists in EPT twins, with females having lower risk than males and opposite-sex pairs having lower risk than same-sex pairs.
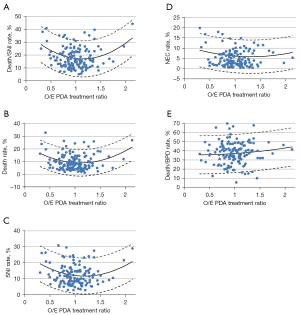
A major controversy in the neonatal field is related to the treatment of PDA. Due to the wide variety of reported outcomes with PDA, several units have adopted a practice of not treating at all, whereas other units have actively looked for PDA within the first 24 hours and aggressively managed it. Isayama et al. (31) reviewed 39,096 neonates born at 24 weeks to 28 weeks’ gestation from 139 neonatal units and assessed rates of PDA treatment at individual unit level (Figure 4). The relationship identified a nadir at a ratio of 1.13 with a significant quadratic effect, indicating that both low and high treatment rates were associated with death or severe neurological injury. Thus, having access to such large cohort allowed us to test some associations which are impossible or difficult to evaluate with randomized studies; however, the results are only amenable to epidemiological scrutiny if they can be tested in a large sample.
Training and mentoring
The iNeo collaboration has been successful in mentoring a graduate student, 3 post-doc fellows, and 3 post-MD fellows, and has contributed to 2 PhD thesis chapters. The next goal is to engage in succession planning, as junior leaders from many networks are being supported to develop their skills in leading the individual networks.
Funding support: sources and return on investments
The day-to-day management of the iNeo collaboration is overseen by the iNeo Director, while a Steering Committee comprising one or two members from each country assesses the overall progress of iNeo, evaluates the scientific merits of proposed projects, reviews results, identifies and articulates strengths and limitations of analyses, and recruits and trains junior researchers interested in international neonatal health. The iNeo Coordinating Centre is housed at the Maternal-infant Care Research Centre (MiCare) within the Lunenfeld-Tanenbaum Research Institute at Mount Sinai Hospital, Sinai Health System, Toronto. Each national network coordinating centre prepares local data for processing, extraction, and transfer, and disseminates findings to its respective sites.
Financial support for the iNeo Coordinating Centre was provided by an Applied Research Chair Grant from the Institute of Human Development Child and Youth Health at the Canadian Institutes of Health Research, and the infrastructure of the individual member networks is supported by their own budgets (identified in funding opportunities). The member network coordinating centres also act as local training sites for trainees in health services research in neonatal-perinatal medicine. In order to foster a true international collaboration, the data collected and housed at the iNeo Coordinating Centre are available to all iNeo member networks and iNeo-affiliated investigators. We have obtained ethics/regulatory approval or its equivalent from the local granting agencies to allow for de-identified data to be collated and sent to the iNeo Coordinating Centre. Overall coordination of the project is also approved by the Research Ethics Board at Mount Sinai Hospital in Toronto, Ontario, Canada. The privacy and confidentiality of patient data is in accordance with the Privacy Commissioner’s guidelines. The group meets every month via a video conferencing platform to discuss existing and new proposals, review the results of existing analyses, brainstorm ideas for future projects with respect to the changing landscape of neonatology, and plan their course of action. The collaboration also meets face-to-face each year during the Pediatric Academic Society’s annual meeting to review overall data structure, quality, and governance issues.
Future directions and plans
The next phase in this collaboration will evaluate how neonatal outcomes are associated with neurodevelopmental outcomes and whether variations persist. A recent review by Ding et al. (32) meta-analyzed estimates from different countries and reported that rates of moderate-to-severe neurodevelopmental disability were 42% for infants born at 22 weeks’ gestation, 41% at 23 weeks, 32% at 24 weeks, and 23% at 25 weeks’ gestation. The Effective Perinatal Intensive Care in Europe (EPICE) collaboration has standardized outcome definitions and measures at 2 years corrected age in 15 European regions for infants born <28 weeks’ gestation (33). Rates of neurodevelopmental impairment ranged from 10% to 26%; however, outcome ascertainment was done using parental questionnaires with response rates as low as 47% in certain regions. We recently compared outcomes of EPT neonates in iNeo networks from Australia-New Zealand and Canada and the EPIPAGE cohort in France born during the year 2011. We identified that mortality was higher in the EPIPAGE cohort compared to Canadian cohort, and mortality or moderate-to-severe neurosensory impairment was higher in EPIPAGE compared to both Australia-New Zealand and Canada. There was no difference in neurosensory impairment among survivors. This increase persisted even after considering differences in baseline characteristics and neonatal complications, suggesting the possible contribution of unmeasured factors that could vary by country (e.g., maternal characteristics, health care organization) or diverging philosophies on end-of-life decisions. The most important lesson learned in this exercise was that there are variations in the ways children are assessed in different countries and jurisdictions, such that harmonization of criteria for classifying children with arbitrary cut-offs could be a challenge (34). However, detailed investigation of variations across settings with similar health care delivery systems is desperately needed to learn the reasons for the variations and identify which modifiable factors can be incorporated into future research on quality improvement and clinical practices.
We perceive a few areas of investigation that will serve as a springboard into the next phase of this incredibly successful collaborative: these include infection (predisposing factors and preventive factors), surgical/lethal necrotizing enterocolitis, infant growth trajectories and their influence on outcomes, comparison and harmonization of infant neurodevelopmental outcomes across countries or networks, and finally, outcomes of neonates with fairly common congenital anomalies.
Challenges: current and future
The collaboration has accomplished neonatal data harmonization, except for our work on sepsis and necrotizing enterocolitis, which is ongoing. Data harmonization for neurodevelopmental outcomes could be a challenge and would require a different set of knowledge users and decision makers from each network. We have begun the initial work in this area, and we are confident we will be able to identify common grounds as we have done previously. Accomplishing this next phase of work will require additional funding for the next five years and the collaboration is currently exploring various ways to identify and secure this support.
Acknowledgments
The authors gratefully acknowledge the diligent work of all site investigators and data abstractors of the networks participating in the International Network for Evaluating Outcomes of Neonates (iNeo) consortium. A list of participating iNeo investigators and their affiliations is provided below. We thank Heather McDonald Kinkaid, PhD, a scientific writer at the Maternal-infant Care Research Centre (MiCare) at Mount Sinai Hospital in Toronto, Ontario, Canada, for editorial support in preparing this manuscript; and other MiCare staff for statistical and organizational support.
iNeo Investigators and Affiliations: ANZNN (Australian and New Zealand Neonatal Network): Kei Lui* Chair of ANZNN. Flinders Medical Centre, SA: Peter Marshall. Gold Coast University Hospital, QLD: Peter Schmidt. Blacktown District Hospital, NSW: Anjali Dhawan*. John Hunter Children’s Hospital, NSW: Larissa Korostenski, Javeed Travadi*. King Edward Memorial and Perth Children’s Hospitals, WA: Mary Sharp, Andy Gill*, Jane Pillow*. Liverpool Hospital, NSW: Jacqueline Stack. Mater Mothers’ Hospital, QLD: Pita Birch, Karen Nothdurft*. Neonatal Retrieval Emergency Service Southern Queensland, QLD: Lucy Cooke*. Mercy Hospital for Women, VIC: Dan Casalaz, Jim Holberton*. Monash Medical Centre, VIC: Alice Stewart. Nepean Hospital, NSW: Lyn Downe. Paediatric Infant Perinatal Emergency Retrieval (VIC): Michael Stewart. NSW Newborn & Paediatric Emergency Transport Service: Andrew Berry. Royal Children’s Hospital, VIC: Rod Hunt*. Royal Darwin Hospital, NT: Peter Morris. Royal Hobart Hospital, Tasmania: Tony De Paoli. Royal Hospital for Women, NSW: Kei Lui*, Srinivas Bolisetty. Royal North Shore Hospital, NSW: Mary Paradisis. Royal Prince Alfred Hospital, NSW: Mark Greenhalgh. Royal Brisbane and Women’s Hospital, QLD: Pieter Koorts. Royal Women’s Hospital, VIC: Carl Kuschel, Sue Jacobs, Lex Doyle. SAAS MedSTAR Emergency Medical Retrieval Services: John Craven. Sydney Children’s Hospital, NSW: Andrew Numa. The Canberra Hospital, ACT: Hazel Carlisle. The Children’s Hospital at Westmead, NSW: Nadia Badawi, Himanshu Popat. The Townsville Hospital, QLD: Guan Koh. Neonatal Emergency Transport Service of Western Australia: Jonathan Davis. Westmead Hospital, NSW: Melissa Luig. Women’s & Children’s Hospital, SA: Bevan Headley, Chad Andersen*. Australian College of Neonatal Nurses: Linda Ng*. National Perinatal Epidemiology and Statistics Unit, University of New South Wales: Georgina Chambers*. New Zealand: Christchurch Women’s Hospital: Nicola Austin, Adrienne Lynn. University of Otago, Christchurch: Brian Darlow. Dunedin Hospital: Liza Edmonds. Middlemore Hospital: Lindsay Mildenhall. Auckland City Hospital: Mariam Buksh, Malcolm Battin*. Waikato Hospital: Jutta van den Boom. Wellington Women’s Hospital: Vaughan Richardson. Whangarei Hospital: David Barker*. Neonatal Nurses College Aotearoa: Barbara Hammond*. Singapore: KK Women’s and Children’s Hospital, Singapore: Victor Samuel Rajadurai*. Hong Kong: Prince of Wales Hospital: Simon Lam. United Christian Hospital: Genevieve Fung. * denotes the ANZNN Executive Committee.
CNN (Canadian Neonatal Network): Prakesh S Shah, MD, MSc (Director, Canadian Neonatal Network and Site Investigator), Mount Sinai Hospital, Toronto, Ontario; Marc Beltempo, MD, (Associate Director, Canadian Neonatal Network and Site Investigator), Montreal Children’s Hospital at McGill University Health Centre, Montréal, Québec; Jaideep Kanungo, MD, Victoria General Hospital, Victoria, British Columbia; Joseph Ting, MD, British Columbia Women’s Hospital, Vancouver, British Columbia; Zenon Cieslak, MD, Royal Columbian Hospital, New Westminster, British Columbia; Rebecca Sherlock, MD, Surrey Memorial Hospital, Surrey, British Columbia; Ayman Abou Mehrem, MD, Foothills Medical Centre, Calgary, Alberta; Jennifer Toye, MD, and Khalid Aziz, MBBS, Royal Alexandra Hospital, Edmonton, Alberta; Carlos Fajardo, MD, Alberta Children’s Hospital, Calgary, Alberta; Jaya Bodani, MD, Regina General Hospital, Regina, Saskatchewan; Lannae Strueby, MD, Royal University Hospital, Saskatoon, Saskatchewan; Mary Seshia, MBChB, and Deepak Louis, MD, Winnipeg Health Sciences Centre, Winnipeg, Manitoba; Ruben Alvaro, MD, St. Boniface General Hospital, Winnipeg, Manitoba; Amit Mukerji, MD, Hamilton Health Sciences Centre, Hamilton, Ontario; Orlando Da Silva, MD, MSc, London Health Sciences Centre, London, Ontario; Mohammad Adie, MD, Windsor Regional Hospital, Windsor, Ontario; Kyong-Soon Lee, MD, MSc, Hospital for Sick Children, Toronto, Ontario; Eugene Ng, MD, Sunnybrook Health Sciences Centre, Toronto, Ontario; Brigitte Lemyre, MD, The Ottawa Hospital, Ottawa, Ontario; Thierry Daboval, MD, Children’s Hospital of Eastern Ontario, Ottawa, Ontario; Faiza Khurshid, MD, Kingston General Hospital, Kingston, Ontario; Ermelinda Pelausa, MD, Jewish General Hospital, Montréal, Québec; Keith Barrington, MBChB, Anie Lapoint, MD, and Guillaume Ethier, NNP, Hôpital Sainte-Justine, Montréal, Québec; Christine Drolet, MD, and Bruno Piedboeuf, MD, Centre Hospitalier Universitaire de Québec, Sainte Foy, Québec; Martine Claveau, MSc, LLM, NNP, Montreal Children’s Hospital at McGill University Health Centre, Montréal, Québec; Marie St-Hilaire, MD, Hôpital Maisonneuve-Rosemont, Montréal, Québec; Valerie Bertelle, MD, and Edith Masse, MD, Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Québec; Roderick Canning, MD, Moncton Hospital, Moncton, New Brunswick; Hala Makary, MD, Dr. Everett Chalmers Hospital, Fredericton, New Brunswick; Cecil Ojah, MBBS, and Luis Monterrosa, MD, Saint John Regional Hospital, Saint John, New Brunswick; Julie Emberley, MD, Janeway Children’s Health and Rehabilitation Centre, St. John’s, Newfoundland; Jehier Afifi, MB BCh, MSc, IWK Health Centre, Halifax, Nova Scotia; Andrzej Kajetanowicz, MD, Cape Breton Regional Hospital, Sydney, Nova Scotia; Shoo K Lee, MBBS, PhD (Chairman, Canadian Neonatal Network), Mount Sinai Hospital, Toronto, Ontario.
FinMBR (Finnish Medical Birth Register): Marjo Metsäranta, MD, Helsinki University Hospital, Helsinki; Liisa Lehtonen, MD, Turku University Hospital, Turku; Outi Tammela, MD, Tampere University Hospital, Tampere; Ulla Sankilampi, MD, Kuopio University Hospital, Kuopio; Timo Saarela, MD, Oulu University Hospital, Oulu.
INN (Israel Neonatal Network): Iris Morag, MD, Assaf Harofeh Medical Center, Omer Globus MD, Assuta Hospital, Ashdod Tzrifin; Shmuel Zangen, MD, Barzilai Medical Center, Ashkelon; Tatyana Smolkin, MD, Baruch Padeh Medical Center, Poriya; Francis Mimouni, MD, Bikur Cholim Hospital, Jerusalem; Arieh Riskin, MD, Bnai Zion Medical Center, Haifa; Karen Lavie-Nevo, MD, Carmel Medical Center, Haifa; Zipora Strauss, Chaim Sheba Medical Center, Ramat Gan; Clari Felszer, MD, Emek Medical Center, Afula; Hussam Omari, MD, French Saint Vincent de Paul Hospital, Nazareth; Smadar Even Tov-Friedman, MD, Hadassah University Hospital-Ein Karem, Jerusalem; Smadar Eventov-Friedman, MD, Hadassah University Hospital-Har Hazofim, Jerusalem; Michael Feldman, MD, Hillel Yaffe Medical Center, Hadera; Nizar Saad, MD, Holy Family (Italian) Hospital, Nazareth; Orna Flidel-Rimon, MD, Kaplan Medical Center, Rehovot; Aryeh Simmonds, MD, Laniado Hospital, Netanya; Daniel Lubin, MD, Mayanei Hayeshua Medical Center, Bnei Brak; Sofia Bauer, MD, Meir Medical Center, Kfar Saba; Amir Kugelman, MD, Rambam Medical Center; Eric Shinwell, MD, Rivka Ziv Medical Center, Safed; Gil Klinger, MD, Schneider Children’s Medical Center of Israel, Rabin Medical Center (Beilinson Campus), Petah Tikva; Yousif Nijim, MD, Scottish (EMMS) Hospital, Nazareth; Alona Bin-Nun, MD, Shaare-Zedek Medical Center, Jerusalem; Eilon Shani, MD, Soroka Medical Center, Beersheba; Dror Mandel, MD, Sourasky Medical Center, Tel Aviv; Vered Fleisher-Sheffer, MD,Western Galilee Medical Center, Nahariya; Anat Oron, MD, Wolfson Medical Center, Holon; Lev Bakhrakh, MD, Yoseftal Hospital, Eilat.
NRNJ (Neonatal Research Network of Japan): Masato Mizushima, MD, Sapporo City Hospital, Sapporo, Hokkaido; Masaru Shirai, MD, Asahikawa Kosei Hospital, Asahikawa, Hokkaido; Toru Ishioka, MD, Engaru Kosei Hospital, Engaru, Hokkaido; Shosuke Kane, MD, Kushiro Red Cross Hospital, Kushiro, Hokkaido; Takashi Nasu, MD, Obihiro Kosei Hospital, Obihiro, Hokkaido; Nobuhiro Takahashi, MD, Tenshi Hospital, Sapporo, Hokkaido; Ayumu□Noro, MD, JCHO Hokkaido Hospital, Sappro, Hokkaido; Toshihiko Mori, MD, NTT East Sappro Hospital, Sapporo, Hokkaido; Tatsuro Satomi, MD, Nikko Kinen Hospital, Sapporo, Hokkaido; Eiki Nakamura, MD, Nayoro City Hospital, Nayoro, Hokkaido; Masaki Kobayashi, MD, Sapporo Prefecture Medical University, Sapporo, Hokkaido; Ken Nagaya, MD, Asahikawa Medical University, Asahikawa, Hokkaido; Tomofumi Sato, MD, Aomori Prefecture Central Hospital, Aomori, Aomori; Genichiro Sotodate, MD, Iwate Medical University, Morioka, Iwate; Toru Huchimukai, MD, Iwate Prefecture Ohfunato Hospital, Ofunato, Iwate; Mari Ishii, MD, Iwate Prefecture Kuji Hospital, Kuji, Iwate; Takahide Hosokawa, MD, Iwate Prefecture Ninohe Hospital, Ninohe, Iwate; Masatoshi Sanjo, MD, Sendai Red Cross Hospital, Sendai, Miyagi; Takushi Hanita, MD, Tohoku University, Sendai, Miyagi; Kazuhiro Arai, MD, Akita Red Cross Hosptai, Akita, Akita; Masato Ito, MD, Akita University, Akita, Akita; Hiroshi Yoshida, MD, Tsuruoka City Shonai Hospital, Tsuruoka, Yamagata; Ayako Sasaki, MD, Yamagata University, Yamagata, Yamagata; Satoshi Watanabe, MD, Yamagata Prefecture Central Hospital, Yamagata, Yamagata; Maki Sato, MD, Fukusima Prefecture Medical University, Fukushima, Fukushima; Takashi Imamura, MD, Takeda General Hospital, Aizuwakamatsu, Fukusima; Tsutomu Ishii, MD, National Fukushima Hospital, Sukagawa, Fukushima; Yayoi Miyazono, MD, Tsukuba University, Tsukuba, Ibaraki; Goro Asada, MD, Tsuchiura Kyodo Hospital, Tsuchiura, Ibaraki; Yoshiya Yukitake, MD, Ibaraki Children’s Hospital, Mito, Ibaraki; Hiroshi Suzuki, MD, Dokkyo Medical University, Shimotugagun, Tochigi; Yumi Kono, MD, Jichi Medical University, Oyama, Tochigi; Yasuaki Kobayashi, MD, Ashikaga Red Cross Hospital, Ashikaga, Tochigi; Kenji Ichinomiya, MD, Gunma Prefecture Children’s Hospital, Maebashi, Gunma; Yasushi Oki, MD, Kiryu Kosei General Hospital, Kiryu, Gunma; Hideaki Fukushima, MD, Ohta General Hospital, Ota, Gunma; Toru Fujiu, MD, Gunma University, Maebashi, Gunma; Tetsuya Kunikata, MD, Saitama Medical University, Iruma, Saitama; Masaki Shimizu, MD, Saitama Prefecture Children’s Hospital, Omiya, Saitama; Toshihiko Nakamura, MD, National Nishisaitama Central Hospital, Tokorozawa, Saitama; Hisanori Sobajima, MD, Saitama Medical University Medical Center, Kawagoe, Satitama; Chika Morioka, MD, Kawaguchi City Medical Center, Kawaguchi, Saitama; Shigeharu Hosono, MD, Jichi Medical University Saitame Medical Center, Omiya, Saitama; Hiroshi Matsumoto, MD, Asahi Central Hospital, Asahi, Chiba; Harumi Otsuka, MD, Chiba City Kaihin Hospital, Chiba, Chiba; Hiroyuki Sato, MD, Kameda General Hospital, Kameda, Chiba; Masahiko Sato, MD, Tokyo Women’s Medical University Yachiyo Medical Center, Yachiyo, Chiba; Naoto Nishizaki, MD, Juntendo University Urayasu Hospital, Urayasu, Chiba; Satoshi Toishi, MD, Narita Red Cross Hospital, Narita, Chiba; Masatoshi Kondo, MD, Tokyo Metropolitan Children’s Medcial Center, Fuchu, Tokyo; Masaki Wada, MD, Tokyo Women’s Medical University, Shinjuku, Tokyo; Shinya Hayashida, MD, Aiiku Hospital, Minato, Tokyo; Ichiro Morioka, MD, Nihon University, Itabashi, Tokyo; Keiji Goishi, MD, National International Medical Center, Shinjuku, Tokyo; Daisuke Haruhara, MD, Tokyo Medical Universtity, Shinjuku, Tokyo; Naoki Ito, MD, Teikyo University, Itabashi, Tokyo; Atsuo Miyazawa, MD, Showa University, Shinagawa, Tokyo; Atsushi Nakao, MD, Japan Red Cross Hospital, Shibuya, Tokyo; Yuji Ito, MD, National Center for Child Health and Development, Setagaya, Tokyo; Ken Masunaga, MD, Tokyo Metropolitan Otsuka Hospital, Toshima, Tokyo; Riki Nishimura, MD, Tokyo University, Bunkyo, Tokyo; Hitoshi Yoda, MD, Toho University, Ota, Tokyo; Isaku Omori, MD, Tokyo Metropolitan Bokuto Hospital, Sumida, Tokyo; Masahiro Kobayashi, MD, Tokyo Jikei Medical University, Minatoku, Tokyo; Atsuko Taki, MD, Tokyo Medical and Dental University, Bunkyo, Tokyo; Rinshu Shimabukuro, MD, Saint Luku Hospital, Chuo, Tokyo; Hiromichi Shoji, MD, Juntendo University, Bunkyo, Tokyo; Kyone Ko, MD, Sanikukai Hospital, Sumida, Tokyo; Sakae Kumasaka, MD, Katsushika Red Cross Hospital, Katsushika, Tokyo; Daisuke Nishi, MD, Yokohama Rosai Hospital, Yokohama, Kanagawa; Kazuo Seki, MD, Yokohama City Universtiy Medical Center, Yokohama, Kanagawa; Isamu Hokuto, MD, Marianna Medical University, Kawasaki, Kanagawa; Katsuaki Toyoshima, MD, Kanagawa Children’s Medical Center, Yokohama, Kanagawa; Keiji Suzuki, MD, Tokai University, Isehara, Kanagawa; Hidehiko Nakanishi, MD, Kitazato University, Sagami, Kanagawa; Rika Kidu, MD, Yokosuka Kyosai Hospital, Yokosuka, Kanagawa; Kanji Ogo, MD, Odawara City Hospital, Odawara, Kanagawa; Yoshio Shima, MD, Nippon Medical School Musashi Kosugi Hospital, Kawasaki, Kanagawa; Daisuke Ogata, MD, Yokohama City Hospital, Yokohama, Kanagawa; Kuriko Nakamura, MD, Saiseikai Eastern Yokohama Hospital, Yokohama, Kanagawa; Ayako Fukuyama, MD, Yokohama Medical Center, Yokohama, Kanagawa; Atsuchi Nemoto, MD, Yamanashi Prefecture Central Hospital, Kofu, Yamanashi; Takehiko Hiroma, MD, Nagano Children’s Hospital, Azumino, Nagano; Yukihide Miyosawa, MD, Shinshu University, Matsumoto, Nagano; Yosuke Shima, MD, Iida City Hospital, Iida, Nagano; Akira Shimazaki, MD, National Shinshu Ueda Medical Center, Ueda, Nagano; Tatsuya Yoda, MD, Saku General Hospital, Saku, Nagano; Tohei Usuda, MD, Nigata University, Niigata, Niigata; Gen Kuratsuji, MD, Niigata Central Hospital, Niigata, Niigata; Yoshhisa Nagayama, MD, Niigata City Hospital, Niigata, Niigata; Rei Kobayashi, MD, Nagaoka Red Cross Hospital, Nagaoka, Niigata; Hiroaki Imamura, MD, Koseiren Takaoka Hospital, Takaoka, Toyama; Takeshi Hutani, MD, Toyama Prefectural Central Hospital, Toyama, Toyama; Taketoshi Yoshida, MD, Toyama University, Toyama, Toyama; Yasuhisa Ueno, MD, Ishikawa Prefectural Central Hospital, Kanazawa, Ishikawa; Azusa Kobayashi, MD, Kanazawa Medical University, Kanazawa, Kanazawa; Kazuhide Ohta, MD, Kanazawa Medcial Center, Kanazawa, Kanazawa; Ritsuyo Taguchi, MD, Fukui Prefectural Hospital, Fukui, Fukui; Takashi Okuno, MD, Fukui University, Fukui, Fukui; Yoshinori Kono, MD, Gifu Prefectural Medical Center, Gifu, Gifu; Takashi Tachibana, MD, Oogaki City Hospital, Oogaki, Gifu; Yasushi Uchida, MD, National Nagara Medical Center, Nagara, Gifu; Shinji Usui, MD, Takayama Red Cross Hospital, Takayama, Gifu; Shigeru Oki, MD, Seirei Hamamatsu Hospital, Hamamatsu, Shizuoka; Taizo Ueno, MD, Shizuoka Saiseikai Hospital, Shizuoka, Shizuoka; Yusuke Nakazawa, MD, Shizuoka Children’s Hospital, Shizuoka, Shizuoka; Akira Oishi, MD, Hamamatsu Medical University, Hamamatsu, Shizuoka; Tokuso Murabayashi, MD, Numazu City Hospital, Numazu, Shizuoka; Takuya Oshima, MD, Yaizu City Hospital, Yaizu, Shizuoka; Mitsuhiro Ito, MD, Fujieda City Hospital, Fujieda, Shizuoka; Taihei Tanaka, MD, Nagoya Red Cross Daini Hospital, Nagoya, Aichi; Ryo Tanaka, MD, Nagoya University, Nagoya, Aichi; Makoto Oshiro, MD, Nagoya Red Cross Daiici Hospital, Nagoya, Aichi; Yasunori Koyama, MD, Toyohashi City Hospital, Toyohashi, Aichi; Takahiro Muramatsu, MD, Nagoya City Seibu Medical Cneter, Nagoya, Aichi; Masashi Miyata, MD, Fujita Medical University, Nagoya, Aichi; Yuichi Kato, MD, Anjokosei Hospital, Anjo, Aichi; Kuniko Ieda, MD, Koritsu Tosei Hospital, Toyota, Aichi; Toshiyuki Ono, MD, Komaki City Hospital, Komaki, Aichi; Hikaru Yamamoto, MD, Toyota Memorial Hospital, Toyota, Aichi; Masashi Hayashi, MD, Okazaki City Hospital, Okazaki, Aichi; Osamu Shinohara, MD, Handa City Hospital, Handa, Aichi; Koji Takemoto, MD, Konankosei Hospital, Kona, Aichi; Osuke Iwata, MD, Nogoya Chity University, Nagoya, Aichi; Hiroshi Takeshita, MD, Aichi Medical University, Nagoya, Aichi; Hiroshi Uchizono, MD, National Mie Cnetral Medical Center, Tsu, Mie; Naoki Kamata, MD, Ise Red Cross Hospital, Ise, Mie; Kanemasa Maki, MD, Yokkaichi City Hospital, Yokkaichi, Mie; Kenji Nakamura, MD, Otsu Red Cross Hospital, Otsu, Shiga; Takahide Yanagi, MD, Shiga Medical University, Otsu, Shiga; Masahito Yamamoto, MD, Nagahama Red Cross Hospital, Nagahama, Shiga; Jitsuko Ohira, MD, Uji Tokushukai Hospital, Uji, Kyoto; Toru Yamakawa, MD, Japan Baptist Hospital, Kyoto, Kyoto; Kogoro Iwanaga, MD, Kyoto University, Kyoto, Kyoto; Daisuke Kinoshita, MD, Kyoto Red Cross Daiichi Hospital, Kyoto, Kyoto; Hiroshi Komatsu, MD, National Maizuru Medical Center, Maizuru, Kyoto; Shinsuke Adachi, MD, Fukuchiyama City Hospital, Fukuchiyama, Kyoto; Ryuji Hasegawa, MD, Kyoto Prefecture Medical University, Kyoto, Kyoto; Kozue Shiomi, MD, Kyoto City Hospital, Kyoto, Kyoto; Koji Nozaki, MD, Mitubishi Kyoto Hospital, Kyoto, Kyoto; Hiroyuki Ito, MD, Yodogawa Christian Hospital, Osaka, Osaka; Yosuke Imanishi, MD, Osaka Women’s and Children’s Hospital, Izumi, Osaka; Hidetoshi Taniguchi, MD, Osaka University, Suita, Osaka; Hirotaka Minami, MD, Takatuski General Hospital, Takatsuki, Osaka; Atsushi Ohashi, MD, Kansai Medical University, Hirakata, Osaka; Hiroyuki Ichiba, MD, Osaka City General Hospital, Osaka, Osaka; Taho Kim, MD, Osaka City Sumiyoshi Hospital, Osaka, Osaka; Kiyoaki Sumi, MD, Aizenbashi Hospital, Osaka, Osaka; Yasuyuki Tokunaga, MD, Toyonaka City Hospital, Toyonaka, Osaka; Reiko Negi, MD, National Cerebral and Cardiovascular Center, Suita, Osaka; Hiroshi Mizumoto, MD, Kitano Hospital, Osaka, Osaka; Satoru Ogawa, MD, Saiseikai Suita Hospital, Suita, Osaka; Akihiro Takatera, MD, Chifune Hospital, Osaka, Osaka; Masahiko Kai, MD, Bell Land General Hospital, Sakai, Osaka; Hiroshi Sumida, MD, Rinku General Hospital, Izumisano, Osaka; Misao Yoshii, MD, Osaka Red Crsoo Hospital, Osaka, Osaka; Yae Michinomae, MD, Yao City Hospital, Yao, Osaka; Ryoko Yoshinare, MD, Hannan Central Hospital, Hannan, Osaka; Yoshio Kusumoto, MD, Osaka General Medical Center, Osaka, Osaka; Satoshi Onishi, MD, Osaka City University, Osaka, Osaka; Seiji Yoshimoto, MD, Kobe Children’s Hospital, Kobe, Hyogo; Kazumichi Fujioka, MD, Kobe University, Kobe, Hyogo; Takeshi Morisawa, MD, Kakogawa City Hospital, Kakogawa, Hyogo; Masayuki Yamane, MD, Saiseikai Hyogo Hospital, Kobe, Hyogo; Masaru Yamakawa, MD, Kobe City Medical Center Central Hospital, Kobe, Hyogo; Maiko Misaki, MD, Hyogo Medical University, Nishinomiya, Hyogo; Tomoaki Ioroi, MD, Himeji Red Cross Hospital, Himeji, Hyogo; Masaaki Ueda, MD, Toyooka General Hospital, Toyooka, Hyogo; Tamaki Ohashi, MD, Hyogo Prefectural Awaji Medical Center, Sumoto, Hyogo; Toshiya Nishikubo, MD, Nara Prefecture Medical University, Kashiwara, Nara; Ken Kumagaya, MD, Wakayama Prefecture Medical University, Wakayama, Wakayama; Mikio Tsunei, MD, Tottori Prefectural Central Hospital, Tottori, Tottori; Masumi Miura, MD, Tottori University, Yonago, Tottori; Fumihide Kato, MD, Shimane Prefectural Central Hospital, Izumo, Shimane; Yuki Hasegawa, MD, Matue Red Cross Hospital, Matsue, Matsue; Shinichi Watabe, MD, Kurashiki Central Hospital, Kurashiki, Okayama; Moriharu Sugimoto, MD, Tsuyama Central Hospital, Tsuyama, Okayama; Yutaka Kawamoto, MD, Kawasaki Medical University, Kurashiki, Okayama; Misao Kageyama, MD, National Okayama Medical Cneter, Okayama, Okayama; Kei Takemoto, MD, Okayama Red Cross Hospital, Okayama, Okayama; Hiroshi Nishimura, MD, Hiroshima City Central Hospital, Hiroshia, Hiroshima; Rie Fukuhara, MD, Hiroshima Prefectural Hospital, Hiroshia, Hiroshima; Noriaki Ono, MD, Hiroshima University, Hiroshima, Hiroshima; Masahiro Tahara, MD, Tsuchiya General Hospital, Hiroshima, Hiroshima; Shinichiro Miyagawa, MD, National Kure Medical Center, Kure, Hiroshima; Kazumasa Takahashi, MD, Yamaguchi University, Ube, Yamaguchi; Keiko Hasegawa, MD, Yamaguchi Prefecture Medical Center, Hofu, Yamaguchi; Takahiko Saijo, MD, Tokushima University, Tokushima, Tokushima; Takashi Yamagami, MD, Tokushima City Hospital, Tokushima, Tokushima; Tomomasa Terada, MD, Tokushima Prefecture Central Hospital, Tokushima, Tokushima; Kosuke Koyano, MD, Kagawa University, Kida, Kagawa; Toru Kuboi, MD, Shikoku Medical Center for Children and Adults, Zentsuji, Kagawa; Yoichi Kondo, MD, Matsuyama Red Cross Hospital, Matsuyama, Ehime; Shinosuke Akiyoshi, MD, Ehime Prefectural Cntral Hospital, Matsuyama, Ehime; Yusei Nakata, MD, Kochi Health Science Center, Kochi, Kochi; Mitsuaki Unno, MD, Saint Maria Hospital, Kurume, Fukuoka; Toshiharu Hikino, MD, National Kyushu Medical Center, Fukuoka, Fukuoka; Hideaki Harada, MD, Kurume University, Kurume, Fukuoka; Naoko Matsumoto, MD, Kitakyushu City Hospital, Kitakyushu, Fukuoka; Shunsuke Araki, MD, University of Occupational and Environmental Health Japan, Kitakyushu, Fukuoka; Koki Nakamura, MD, Fukuoka University, Fukuoka, Fukuoka; Masayuki Ochiai, MD, Kyushu University, Fukuoka, Fukuoka; Hiroshi Kanda, MD, Iizuka Hospital, Iizuka, Fukuoka; Yoshihiro Sakemi, MD, National Kokura Medical Center, Kitakyushu, Fukuoka; Yasushi Takahata, MD, Fukuoka City Children’s Hospital, Fukuoka, Fukuoka; Toshimitsu Takayanagi, MD, National Saga Hospital, Saga, Saga; Masato Tagawa, MD, Nagasaki University, Nagasaki, Nagasaki; Mikio Aoki, MD, National Nagasaki Medical Cneter, Nagasaki, Nagasaki; Muneichiro Sumi, MD, Saseho City Hospital, Saseho, Nagasaki; Akihiko Kawase, MD, Kumamoto City Hospital, Kumamoto, Kumamoto; Masanori Iwai, MD, Kumamoto University, Kumamoto, Kumamoto; Koichi Iida, MD, Oita Prefectural Hospital, Oita, Oita; Naoki Fukushima, MD, Almeida Memorial Hospital, Oita, Oita; Mitsushi Goshi, MD, Nakatsu City Hospital, Nakatsu, Oita; Yuki Kodama, MD, Miyazaki University, Miyazaki, Miyazaki; Shuichi Yanagibe, MD, National Miyakonojo Hospital, Miyakonojo, Miyazaki; Chie Ishihara, MD, Kagosima City Hospital, Kagoshima, Kagoshima; Yuko Maruyama, MD, Imakyure General Hospital, Kagoshima, Kagoshima; Tatsuo Oshiro, MD, Okinawa Prefectural Nanbu Medcial Center/Nanbu Child Medical Center, Shimajiri, Okinawa; Yoriko Kisato, MD, Okinara Prefectural Central Hospital, Uruma, Okinawa; Asao Yara, MD, Naha City Hospital, Naha, Okinawa; Kazuhiko Nakasone, MD, Okinawa Red Cross Hospital, Naha, Okinawa.
SEN1500 (Spanish Neonatal Network): Alejandro Avila-Alvarez, MD, and José Luis Fernandez-Trisac, MD, Complexo Hospitalario Universitario De A Coruña, A Coruña; Mª Luz Couce Pico, MD, and María José Fernández Seara, MD, Hospital Clínico Universitario de Santiago, Santiago de Compostela; Andrés Martínez Gutiérrez, MD, Complejo Hospitalario Albacete, Albacete; Carolina Vizcaíno , MD, Hospital General Universitario de Elche, Alicante; María Gonzalez Santacruz, MD, and Honorio Sánchez Zaplana, MD, Hospital General Universitario de Alicante, Alicante; Belén Fernández Colomer, MD, and José Enrique García López, MD, Hospital Universitario Central de Asturias, Oviedo, Asturias; Rafael García Mozo, MD, and M. Teresa González Martínez, MD, Hospital Universitario de Cabueñes, Gijón, Asturias; Mª Dolores Muro Sebastián, MD, and Marta Balart Carbonell, MD, Clínica Corachán, Barcelona; Joan Badia Barnusell, MD, and Mònica Domingo Puiggròs, MD, Corporacio Parc Taulí, Sabadell, Barcelona; Josep Figueras Aloy, MD, and Francesc Botet Mussons, MD, Hospital Clínic de Barcelona, Barcelona; Israel Anquela Sanz, MD, Hospitalario Granollers, Barcelona; Gemma Ginovart Galiana, MD, H. De La Santa Creu I Sant Pau, Barcelona; W. Coroleu, MD, Hospital Universitari Germans Trias I Pujol, Barcelona; Martin Iriondo, MD, Hospital Sant Joan de Déu, Barcelona; Laura Castells Vilella, MD, Hospital General de Cataluña, Barcelona; Roser Porta, MD, Institute Dexeus, Barcelona; Xavier Demestre, MD, and Silvia Martínez Nadal, MD, Scias-Hospital Barcelona; Cristina de Frutos Martínez, MD, Hospital Universitario de Burgos, Burgos; María Jesús López Cuesta, MD, H. San Pedro de Alcántara, Cáceres; María Victoria Ramos Ramos, MD, and María Teresa de Benito Guerra, MD, Hospital Jerez, Cádiz; Antonio Segado Arenas MD, and Almudena Alonso, MD, Hospital Universitario Puerta Del Mar, Cádiz; Ramón Aguilera Olmos, MD, Hospital General de Castellón, Castellón; Miguel A. García Cabezas, MD, and Mª Dolores Martínez Jiménez, MD, Hospital General Universitario de Ciudad Real, Ciudad Real; Mª Pilar Jaraba Caballero, MD, and Mª Dolores Ordoñez Díaz, MD, Hospital Universitario Reina Sofía, Córdoba; Alberto Trujillo Fagundo, MD, and Lluis Mayol Canals, MD, Hospital Universitari Dr. Josep Trueta, Girona; Fermín García-Muñoz Rodrigo, MD, and Lourdes Urquía Martí, MD, H.M.I. Las Palmas, Las Palmas, Gran Canaria; María Fernanda Moreno Galdo , MD, and José Antonio Hurtado Suazo, MD, Hospital Universitario Virgen De Las Nieves, Granada; Eduardo Narbona López, and José Uberos Fernández, MD, Hospital Universitario San Cecilio, Granada; Miguel A Cortajarena Altuna, MD, and Oihana Muga Zuriarrain Hospital, MD, Donostia, Gipuzkoa; David Mora Navarro, MD, Hospital Juan Ramón Jiménez, Huelva; Mª Yolanda Ruiz del Prado, MD, and Inés Esteban Díez, MD, Hospital San Pedro, Logroño, La Rioja; María Teresa Palau Benavides, MD, and Santiago Lapeña, MD, Hospital de León, León, León; Teresa Prada, MD, Hospital del Bierzo, Ponferrada, León; Eduard Soler Mir, MD, Hospital Arnau De Vilanova, Lleida; Araceli Corredera Sánchez, MD, Enrique Criado Vega, MD, Náyade del Prado, MD, and Cristina Fernández, MD, Hospital Clínico San Carlos, Madrid; Lucía Cabanillas Vilaplana, MD, and Irene Cuadrado Pérez, MD, Hospital Universitario De Getafe, Madrid; Laura Domingo Comeche, MD, Hospital Universitario de Fuenlabrada, Fuenlabrada, Madrid; Carmen González Armengod, MD, and Carmen Muñoz Labián, MD, Hospital Universitario Puerta De Hierro, Majadahonda, Madrid; Mª José Santos Muñoz, MD and Ersilia González Carrasco, Hospital Severo Ochoa, Leganés, Madrid; Dorotea Blanco Bravo, MD, and Susana Zeballos, MD, Hospital Gregorio Marañón, Madrid; Mª Dolores Elorza Fernández, MD, Celia Díaz González, MD, and Susana Ares Segura, MD, H.U. La Paz, Madrid; Manuela López Azorín, MD, Hospital Universitario Quirón salud, Madrid; Ana Belén Jimenez MD, Hospital Universitario Fundación Jiménez Díaz, Madrid; Tomás Sánchez-Tamayo, MD, and Elías Tapia Moreno, MD, Hospital Carlos Haya, Málaga; José María Lloreda García, MD, Hospital Universitario Santa Lucia De Cartagena, Murcia; Concepción Goñi Orayen, MD, Hospital Virgen Del Camino De Pamplona, Pamplona, Navarra; Maria Angeles Martinez Fernandez MD, Complexo Hospitalario Pontevedra, Pontevedra; María Suárez Albo, MD, and Eva González Colmenero, MD, Hospital Xeral De Vigo, Pontevedra; Elena Pilar Gutiérrez González, MD, and Beatriz Vacas del Arco, MD, Hospital Universitario de Salamanca, Salamanca; Josefina Márquez Fernández, MD, and Laura Acosta Gordillo, MD, Hospital Valme, Sevilla; Mercedes Granero Asensio, MD, Hospital Virgen De La Macarena, Sevilla; Carmen Macías Díaz, MD, Hospital Universitario Virgen Del Rocío, Sevilla; Mar Albújar, MD, Hospital Universitari de Tarragona Joan XXIII, Tarragona; Pedro Fuster Jorge. MD, Hospital Universitario De Canarias, San Cristóbal de La Laguna, Santa Cruz de Tenerife; Sabina Romero, MD, and Mónica Rivero Falero, MD, Hospital Universitario Nuestra Señora De Candelaria, Santa Cruz de Tenerife; Ana Belén Escobar Izquierdo, Hospital Virgen De La Salud, Toledo; Javier Estañ Capell, MD, Hospital Clinico Universitario De Valencia, Valencia; Mª Isabel Izquierdo Macián, MD, Hospital Universitari La Fe, Valencia; Mª Mar Montejo Vicente, MD, and Raquel Izquierdo Caballero, MD, Hospital Universitario Río Hortega, Valladolid; Mª Mercedes Martínez, MD, and Aintzane Euba, MD, Hospital de Txagorritxu, Vitoria-Gasteiz; Amaya Rodríguez Serna, MD, and Juan María López de Heredia Goya, MD, Hospital de Cruces, Baracaldo; Alberto Pérez Legorburu, MD, and Ana Gutiérrez Amorós, MD, Hospital Universitario de Basurto, Vizcaya; Víctor Manuel Marugán Isabel, MD, and Natalio Hernández González, MD, Hospital Virgen De La Concha - Complejo Asistencial De Zamora, Zamora; Segundo Rite Gracia, MD, Hospital Miguel Servet, Zaragoza; Mª Purificación Ventura Faci, MD, and Mª Pilar Samper Villagrasa, MD, Hospital Clínico Universitario Lozano Blesa, Zaragoza.
SNQ (Swedish Neonatal Quality Register): Zeljka Mustapic, MD, Södra Älvsborgs Sjukhus, Borås; Katarina Strand Brodd, MD, Mälarsjukhuset, Eskilstuna; Andreas Odlind, MD, Falu Lasarett, Falun; Per Friskopp, MD, Gällivare Sjukhus, Gällivare; Sofia Arwehed, MD, Gävle Sjukhus, Gävle; Ola Hafström, MD, SU/Östra, Göteborg; Anna Kasemo, MD, Länssjukhuset, Halmstad; Karin Nederman, MD, Helsingborgs Lasarett, Helsingborg; Thomas Hägg, MD, Hudiksvalls Sjukhus, Hudiksvall; Fredrik Ingemarsson, MD, Länssjukhuset Ryhov, Jönköping; Henrik Petersson, MD, Länssjukhuset, Kalmar; Ulrik Lindström, MD, Blekingesjukhuset, Karlskrona; Eva Albinsson, MD, Centralsjukhuset, Karlstad; Bo Selander, MD, Centralsjukhuset, Kristianstad; Thomas Abrahamsson, MD, Universitetssjukhuset, Linköping; Ingela Heimdahl, MD, Sunderby sjukhus, Luleå; Kristbjorg Sveinsdottir, MD, Skånes Universitetssjukhus, Malmö/Lund; Erik Wejryd, MD, Vrinnevisjukhuset, Norrköping; Johanna Kusima-Löfbom, MD, Skellefteå Lasarett, Skellefteå; Maria Katarina Söderberg, MD, Kärnsjukhuset Skaraborg, Skövde; Lars Navér, MD, Karolinska Sjukhuset, Stockholm; Thomas Brune, MD, Södersjuhuset, Stockholm; Jens Bäckström, MD, Länssjukhuset, Sundsvall; Peder Helmersson, MD, Norra Älvsborgs Länssjukhus, Trollhättan; Aijaz Farooqi, MD, Norrlands Universitetssjukhus, Umeå; Erik Normann, MD, Akademiska Barnsjukhuset, Uppsala; Magnus Fredriksson, MD, Visby Lasarett, Visby; Anders Palm, MD, Västerviks Sjukhus, Västervik; Urban Rosenqvist, MD, Centrallasarettet, Västerås; Bengt Walde, MD, Centrallasarettet, Växjö; Linda Resman, MD, Lasarettet, Ystad; Miriam Pettersson, MD, Universitetssjukhuset, Örebro; Christina Ziegel, MD, Örnsköldsviks Sjukhus, Örnsköldsvik; Kari Arhimaa, MD, Östersunds Sjukhus, Östersund.
SwissNeoNet (Swiss Neonatal Network): Mark Adams, PhD (Network coordinator), University Hospital Zurich; Philipp Meyer, MD, and Rachel Kusche, MD, Cantonal Hospital, Children’s Clinic, Aarau; Sven Schulzke, MD, University Children’s Hospital, Basel; M. Ragazzi, San Giovanni Hospital, Bellinzona; Mathias Nelle, MD, University Hospital, Berne; Tilman Humpl, MD, University Hospital, Berne; Mathias Gebauer, MD, Children’s Hospital Wildermeth, Biel; Thomas Riedel, MD, Children’s Hospital, Chur; Bendicht Wagner, MD, Cantonal Hospital, Fribourg; Riccardo E. Pfister, MD, University Hospital (HUG), Geneva; Jean-François Tolsa, MD, and Matthias Roth, MD, University Hospital (CHUV), Lausanne; Martin Stocker, MD, Children’s Hospital, Lucerne; Bernd Erkert, Cantonal Hospital, Muensterlingen; Ikbel El Faleh, MD, Cantonal Hospital, Neuchatel; Andreas Malzacher, MD, Cantonal Hospital, St. Gallen; Bjarte Rogdo, MD, Children’s Hospital, St. Gallen; Lukas Hegi, MD, Cantonal Hospital, Winterthur; Vera Bernet, MD, Spital Zollikerberg, Zollikerberg; Maren Tomaske, Stadtspital Triemli, Zürich; Dirk Bassler, MD, and Romaine Arlettaz, MD, University Hospital (USZ), Zurich; Cornelia Hagmann, MD, University Children’s Hospital, Zurich.
TuscanNN (Tuscany Neonatal Network): Carlo Dani, MD, Careggi University Hospital, Florence, Italy; Patrizio Fiorini, MD, Anna Meyer Children’s University Hospital, Florence, Italy; Paolo Ghirri, MD, University Hospital of Pisa, Pisa, Italy; Barbara Tomasini, MD, University Hospital of Siena, Siena, Italy.
UKNC (United Kingdom Neonatal Collaborative): Anita Mittal, MBChB, Bedford Hospital, Bedford, Bedfordshire; Jonathan Kefas, MBChB, Lister Hospital, Stevenage, Hertfordshire; Anand Kamalanathan, MBChB, Arrowe Park Hospital, Wirral, Merseyside; Michael Grosdenier, MBChB, Leighton Hospital, Crewe, Cheshire; Christopher Dewhurst, MBChB, Liverpool Women’s Hospital, Liverpool, Merseyside; Andreea Bontea, MBChB, Ormskirk District General Hospital, Ormskirk, Lancashire; Delyth Webb, MBChB, Warrington Hospital, Warrington, Cheshire; Ross Garr, MBChB, Whiston Hospital, Prescot, Merseyside; Ahmed Hassan, MBChB, Broomfield Hospital, Chelmsford, Essex; Priyadarshan Ambadkar, MBChB, James Paget Hospital, Gorleston, Norfolk; Mark Dyke, MBChB, Norfolk & Norwich University Hospital, Norwich, Norfolk; Katharine McDevitt, MBChB, Peterborough City Hospital, Peterborough, Cambridgeshire; Glynis Rewitzky, MBChB, Queen Elizabeth Hospital, King’s Lynn, Birmingham, West Midlands; Angela D’Amore, MBChB, Rosie Maternity Hospital, Addenbrookes, Cambridge, Cambridgeshire; P Kamath, MBChB, North Manchester General Hospital, Manchester, Greater Manchester; Paul Settle, MBChB, Royal Bolton Hospital, Bolton, Lancashire; Natasha Maddock, MBChB, Royal Oldham Hospital, Manchester, Greater Manchester; Ngozi Edi-Osagie, MBChB, St Mary’s Hospital, Manchester, Greater Manchester; Christos Zipitis, MBChB, The Royal Albert Edward Infirmary, Wrightington, Greater Manchester; Carrie Heal, MBChB, Stepping Hill Hospital, Stockport, Cheshire; Jacqeline Birch, MBChB, Tameside General Hospital, Ashton-under-Lyne, Lancashire; Abdul Hasib, MBChB, Darent Valley Hospital, Dartford, Kent; Aung Soe, MBChB, Medway Maritime Hospital, Gillingham, Kent; Bushra Abdul-Malik, MBChB, Queen Elizabeth The Queen Mother Hospital, Margate, Kent; Hamudi Kisat, MBChB, Tunbridge Wells Hospital, Tunbridge Wells, Kent; Vimal Vasu, MBChB, William Harvey Hospital, Ashford, Kent; Savi Sivashankar, MBChB, Lancashire Women & Newborn Centre, Burnley, Lancashire; Richa Gupta, MBChB, Royal Preston Hospital, Preston, Lancashire; Chris Rawlingson, MBChB, Victoria Hospital, Blackpool, Blackpool, Lancashire; Tim Wickham, MBChB, Barnet Hospital, Barnet, Hertfordshire; Marice Theron, MBChB, The Royal Free Hospital, Hampstead, London; Giles Kendall, MBChB, University College Hospital, Fitzrovia, London; Aashish Gupta, MBChB, Basildon Hospital, Basildon, Essex; Narendra Aladangady, MBChB, Homerton Hospital, Hackney, London; Imdad Ali, MBChB, Newham General Hospital, Newham, London; Neeraj Jain, MBChB, North Middlesex University Hospital, Edmonton, London; Khalid Mannan, MBChB, Queen’s Hospital, Romford, Essex; Vadivelam Murthy, MBChB, The Royal London Hospital, Whitechapel, London; Caroline Sullivan, MBChB, Whipps Cross University Hospital, Whipps Cross, London; Shu-Ling Chuang, MBChB, Chelsea & Westminster Hospital, Chelsea, London; Tristan Bate, MBChB, Hillingdon Hospital, Hillingdon, London; Lidia Tyszcuzk, MBChB, Queen Charlotte’s Hospital, East Acton, London; Lidia Tyszcuzk, MBChB, St Mary’s Hospital, Westminister, London; Geraint Lee, MBChB, Guy’s & St Thomas’ Hospital, Lambeth, London; Ozioma Obi, MBChB, University Hospital Lewisham, Lewisham, London; John Chang, MBChB, Croydon University Hospital, Croydon, Surrey; Vinay Pai, MBChB, Kingston Hospital, Kingston, London; Charlotte Huddy, MBChB, St George’s Hospital, Wandsworth, London; Salim Yasin, MBChB, St. Helier Hospital, Merton, London; Richard Nicholl, MBChB, Northwick Park Hospital, Brent, London; Poornima Pandey, MBChB, Kettering General Hospital, Kettering, Northhamptonshire; Jonathan Cusack, MBChB, Leicester General Hospital, Leicester, Leicestershire; Venkatesh Kairamkonda, MBChB, Leicester Royal Infirmary, Leicester, Leicestershire; Dominic Muogbo, MBChB, Queen’s Hospital, Burton On Trent, Burton-on-Trent, Staffordshire; Liza Harry, MBChB, Alexandra Hospital, Redditch, Worcestershire; Pinki Surana, MBChB, Birmingham Heartlands Hospital, Birmingham, West Midlands; Penny Broggio, MBChB, City Hospital, Birmingham, West Midlands; Pinki Surana, MBChB, Good Hope Hospital, Birmingham, West Midlands; Liza Harry, MBChB, Worcestershire Royal Hospital, Worcester, Worcestershire; Tilly Pillay, MBChB, New Cross Hospital, Wolverhampton, West Midlands; Sanjeev Deshpande, MBChB, Princess Royal Hospital, Shrewsbury, Shropshire; Mahadevan, MBChB, Russells Hall Hospital, Dudley, West Midlands; Alison Moore, MBChB, Royal Stoke University Hospital, Hartshill, Staffordshire; Porus Bastani, MBChB, The Jessop Wing, Sheffield, South Yorkshire; Mehdi Garbash, MBChB, Darlington Memorial Hospital, Darlington, County Durham; Mithilesh Lal, MBChB, James Cook University Hospital, Middlesborough, North Yorkshire; Majd Abu-Harb, MBChB, Sunderland Royal Hospital, Sunderland, Tyne and Wear; Mehdi Garbash, MBChB, University Hospital Of North Durham, Durham, Durham; Alex Allwood, MBChB, Derriford Hospital, Plymouth, Devon; Michael Selter, MBChB, North Devon District Hospital, Barnstaple, Devon; Paul Munyard, MBChB, Royal Cornwall Hospital, Truro, Cornwall; David Bartle, MBChB, Royal Devon & Exeter Hospital, Exeter, Devon; Siba Paul, MBChB, Torbay Hospital, Torquay, Devon; Graham Whincup, MBChB, Conquest Hospital, St.Leonards-on-sea, East Sussex; Sanghavi Rekha, MBChB, Frimley Park Hospital, Frimley, Surrey; Philip Amess, MBChB, Princess Royal Hospital, Telford, Shropshire; Ben Obi, MBChB, Royal Surrey County Hospital, Guildford, Surrey; Philip Amess, MBChB, Royal Sussex County Hospital, Brighton, East Sussex; Peter Reynolds, MBChB, St Peter’s Hospital, Chertsey, Surrey; Indranil Misra, MBChB, Milton Keynes Foundation Trust Hospital, Milton Keynes, Buckinghamshire; Peter De Halpert, MBChB, Royal Berkshire Hospital, Reading, Berkshire; Sanjay Salgia, MBChB, Stoke Mandeville Hospital, Aylesbury, Buckinghamshire; Rekha Sanghavi, MBChB, Wexham Park Hospital, Slough, Berkshire; Nicola Paul, MBChB, Basingstoke & North Hampshire Hospital, Basingstoke, Hampshire; Abby Deketelaere, MBChB, Dorset County Hospital, Dorchester, Dorset; Minesh Khashu, MBChB, Poole Hospital NHS Foundation Trust, Poole, Dorset; Mark Johnson, MBChB, Princess Anne Hospital, Southampton, Hampshire; Charlotte Groves, MBChB, Queen Alexandra Hospital, Portsmouth, Hampshire; Jim Baird, MBChB, Salisbury District Hospital, Salisbury, Wiltshire; Nick Brennan, MBChB, St Richard’s Hospital, Chichester, West Sussex; Katia Vamvakiti, MBChB, Worthing Hospital, Worthing, West Sussex; John McIntyre, MBChB, Royal Derby Hospital, Derby, Derbyshire; Jennifer Holman, MBChB, Gloucestershire Royal Hospital, Gloucester, Gloucestershire; Stephen Jones, MBChB, Royal United Hospital, Avon, Somerset; Alison Pike, MBChB, Southmead Hospital, Westbury-on-Trym, Bristol; Pamela Cairns, MBChB, St Michael’s Hospital, Bristol, Bristol; Megan Eaton, MBChB, Yeovil District Hospital, Yeovil, Somerset; Karin Schwarz, MBChB, Calderdale Royal Hospital, Halifax, West Yorkshire; David Gibson, MBChB, Pinderfields General Hospital., Dewsbury, West Yorkshire; Lawrence Miall, MBChB, Leeds Neonatal Service, Leeds, Yorkshire; David Gibson, MBChB, Pinderfields General Hospital, Wakefield, West Yorkshire; Krishnamurthy, MBChB, Walsall Manor Hospital, Walsall, West Midlands.
Funding: We received no funding for this manuscript. However, the International Network for Evaluating Outcomes of Neonates (iNeo) has been supported by the Canadian Institutes of Health Research [No. APR-126340 to P.S.S.]. The Australian and New Zealand Neonatal Network is predominantly funded by membership contributions from the participating centers. The Canadian Neonatal Network is supported by a team grant from the Canadian Institutes of Health Research [No. CTP 87518], by the Ontario Ministry of Health and Long-Term Care, and by the participating centers. The Finnish Medical Birth Register is governmentally funded and kept by the National Institute for Health and Welfare (THL). The Israel Neonatal Network very low birth weight infant database is partially funded by the Israel Center for Disease Control and the Ministry of Health. The Neonatal Research Network of Japan is partly funded by a Health Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan. The Spanish Neonatal Network is supported by funds from the Spanish Neonatal Society (SENeo). The Swedish Neonatal Quality Register is funded by the Swedish Government (Ministry of Health and Social Affairs), the body of regional health care providers (County Councils), and the participating units. The Swiss Neonatal Network is partially funded by the participating units in the form of membership fees. The Tuscany Neonatal Network is funded by the Tuscany Region. The United Kingdom Neonatal Collaborative receives no core funding. This research was also supported by Instituto de Investigación Sanitaria Carlos III (Ministry of Science, Innovation and Universities, Kingdom of Spain) [No. FIS17/0131 to M.V.]; and RETICS funded by the PN 2018-2021 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion, and the European Regional Development Fund (ERDF) [No. RD16/0022]; and grants from a regional agreement on clinical research (ALF) between Region Stockholm and Karolinska Institutet [No. 2020-0443 to M.N.]. The funding bodies played no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine for the series “Neonatal Networks for Outcomes Improvement: Evolution, Progress and Future”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-73/coif). The series “Neonatal Networks for Outcomes Improvement: Evolution, Progress and Future” was commissioned by the editorial office without any funding or sponsorship. PSS and SKL served as the unpaid Guest Editors for the series. DB serves as unpaid editorial board member of Pediatric Medicine from October 2020 to September 2022. SKL serves as unpaid editorial board member of Pediatric Medicine from September 2020 to August 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shah PS, Lui K, Reichman B, et al. The International Network for Evaluating Outcomes (iNeo) of neonates: evolution, progress and opportunities. Transl Pediatr 2019;8:170-81. [Crossref] [PubMed]
- Canadian Institutes of Health Research. CIHR Chairs in Reproductive, Child and Youth Health. 2019. Available online: http://www.cihr-irsc.gc.ca/e/46349.html. Accessed June 14 2019.
- Beltempo M, Shah PS. The Importance of Harmonized Databases for Infants Born Extremely Preterm-If You Are Not Counted, You Are Not Accounted. JAMA Netw Open 2021;4:e219709. [Crossref] [PubMed]
- Zhu Z, Yuan L, Wang J, et al. Mortality and Morbidity of Infants Born Extremely Preterm at Tertiary Medical Centers in China From 2010 to 2019. JAMA Netw Open 2021;4:e219382. [Crossref] [PubMed]
- Shah PS, Lehtonen L. Net worth of networks: opportunities and potential. Acta Paediatr 2019;108:1374-6. [Crossref] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. 2010. Available online: https://icd.who.int/browse10/2010/en. Accessed June 18 2019.
- International Health Terminology Standards Development Organization. SNOMED CT, 2019. Available online: https://www.snomed.org/snomed-ct/five-step-briefing. Accessed June 18 2019.
- Shah PS, Lee SK, Lui K, et al. The International Network for Evaluating Outcomes of very low birth weight, very preterm neonates (iNeo): a protocol for collaborative comparisons of international health services for quality improvement in neonatal care. BMC Pediatr 2014;14:110. [Crossref] [PubMed]
- Kelly LE, Shah PS, Håkansson S, et al. Perinatal health services organization for preterm births: a multinational comparison. J Perinatol 2017;37:762-8. [Crossref] [PubMed]
- Shah PS, Lui K, Sjörs G, et al. Neonatal Outcomes of Very Low Birth Weight and Very Preterm Neonates: An International Comparison. J Pediatr 2016;177:144-152.e6. [Crossref] [PubMed]
- Lui K, Lee SK, Kusuda S, et al. Trends in Outcomes for Neonates Born Very Preterm and Very Low Birth Weight in 11 High-Income Countries. J Pediatr 2019;215:32-40.e14. [Crossref] [PubMed]
- Helenius K, Sjörs G, Shah PS, et al. Survival in Very Preterm Infants: An International Comparison of 10 National Neonatal Networks. Pediatrics 2017;140:e20171264. [Crossref] [PubMed]
- Hines D, Modi N, Lee SK, et al. Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr 2017;106:366-74. [Crossref] [PubMed]
- Darlow BA, Lui K, Kusuda S, et al. International variations and trends in the treatment for retinopathy of prematurity. Br J Ophthalmol 2017;101:1399-404. [Crossref] [PubMed]
- Adams M, Bassler D, Darlow BA, et al. Preventive strategies and factors associated with surgically treated necrotising enterocolitis in extremely preterm infants: an international unit survey linked with retrospective cohort data analysis. BMJ Open 2019;9:e031086. [Crossref] [PubMed]
- Persson M, Shah PS, Rusconi F, et al. Association of Maternal Diabetes With Neonatal Outcomes of Very Preterm and Very Low-Birth-Weight Infants: An International Cohort Study. JAMA Pediatr 2018;172:867-75. [Crossref] [PubMed]
- Gemmell L, Martin L, Murphy KE, et al. Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks' gestation. J Perinatol 2016;36:1067-72. [Crossref] [PubMed]
- Shah PS, Kusuda S, Hakansson S, et al. Neonatal outcomes of very preterm or very low birth weight triplets. Pediatrics 2018;142:e20181938. [Crossref] [PubMed]
- Norman M, Håkansson S, Kusuda S, et al. Neonatal Outcomes in Very Preterm Infants With Severe Congenital Heart Defects: An International Cohort Study. J Am Heart Assoc 2020;9:e015369. [Crossref] [PubMed]
- Lui K, Vento M, Modi N, et al. Inter-center variability in neonatal outcomes of preterm infants: A longitudinal evaluation of 298 neonatal units in 11 countries. Semin Fetal Neonatal Med 2021;26:101196. [Crossref] [PubMed]
- Shahroor M, Lehtonen L, Lee SK, et al. Unit-Level Variations in Healthcare Professionals' Availability for Preterm Neonates <29 Weeks' Gestation: An International Survey. Neonatology 2019;116:347-55. [Crossref] [PubMed]
- Lehtonen L, Lee SK, Kusuda S, et al. Family Rooms in Neonatal Intensive Care Units and Neonatal Outcomes: An International Survey and Linked Cohort Study. J Pediatr 2020;226:112-7.e4. [Crossref] [PubMed]
- Seaton SE, Draper ES, Adams M, et al. Variations in Neonatal Length of Stay of Babies Born Extremely Preterm: An International Comparison Between iNeo Networks. J Pediatr 2021;233:26-32.e6. [Crossref] [PubMed]
- Beltempo M, Isayama T, Vento M, et al. Respiratory Management of Extremely Preterm Infants: An International Survey. Neonatology 2018;114:28-36. [Crossref] [PubMed]
- Darlow BA, Vento M, Beltempo M, et al. Variations in Oxygen Saturation Targeting, and Retinopathy of Prematurity Screening and Treatment Criteria in Neonatal Intensive Care Units: An International Survey. Neonatology 2018;114:323-31. [Crossref] [PubMed]
- Helenius K, Morisaki N, Kusuda S, et al. Survey shows marked variations in approaches to redirection of care for critically ill very preterm infants in 11 countries. Acta Paediatr 2020;109:1338-45. [Crossref] [PubMed]
- Shah PS. Neonatal Intensive Care - The Only Constant Is Change. N Engl J Med 2017;376:694-6. [Crossref] [PubMed]
- Martin LJ, Sjörs G, Reichman B, et al. Country-Specific vs. Common Birthweight-for-Gestational Age References to Identify Small for Gestational Age Infants Born at 24-28 weeks: An International Study. Paediatr Perinat Epidemiol 2016;30:450-61. [Crossref] [PubMed]
- Koller-Smith LI, Shah PS, Ye XY, et al. Comparing very low birth weight versus very low gestation cohort methods for outcome analysis of high risk preterm infants. BMC Pediatr 2017;17:166. [Crossref] [PubMed]
- Gagliardi L, Rusconi F, Reichman B, et al. Neonatal outcomes of extremely preterm twins by sex pairing: an international cohort study. Arch Dis Child Fetal Neonatal Ed 2021;106:17-24. [Crossref] [PubMed]
- Isayama T, Kusuda S, Reichman B, et al. Neonatal Intensive Care Unit-Level Patent Ductus Arteriosus Treatment Rates and Outcomes in Infants Born Extremely Preterm. J Pediatr 2020;220:34-39.e5. [Crossref] [PubMed]
- Ding S, Lemyre B, Daboval T, et al. A meta-analysis of neurodevelopmental outcomes at 4-10 years in children born at 22-25 weeks gestation. Acta Paediatr 2019;108:1237-44. [Crossref] [PubMed]
- Draper ES, Zeitlin J, Manktelow BN, et al. EPICE cohort: two-year neurodevelopmental outcomes after very preterm birth. Arch Dis Child Fetal Neonatal Ed 2020;105:350-6. [Crossref] [PubMed]
- Ding S, Mew EJ, Chee-A-Tow A, et al. Neurodevelopmental outcome descriptions in cohorts of extremely preterm children. Arch Dis Child Fetal Neonatal Ed 2020;105:510-9. [Crossref] [PubMed]
Cite this article as: Shah PS, Isayama T, Helenius KK, San Feliciano L, Beltempo M, Bassler D, Håkansson S, Rusconi F, Modi N, Battin M, Vento M, Adams M, Lehtonen L, Norman M, Kusuda S, Reichman B, Lui K, Lee SK; the International Network for Evaluating Outcomes of Neonates (iNeo). International network for evaluating outcomes of neonates: outputs and future directions. Pediatr Med 2022;5:40.

