Clinical management of epilepsy associated with low-grade glioma and literature review
Introduction
Epilepsy, a neurological disorder characterized by recurrent epileptic seizures, is a common symptom and often the only symptom presenting for brain tumors (1). The incident rate of epilepsy associated with brain tumors (EAT) differs based on the pathological type (2,3). Low-grade glioma (LGG), a common type of brain glioma, often presents with seizure in the course of the disease, and seizure is often the first or only symptom of LGG (4). LGG-related epilepsy is usually refractory to traditional or new generation anti-epilepsy drugs (AEDs), and as such is termed drug-resistant epilepsy (DRE) (5). As LGG is slow-growing, the prognosis is generally favorable, so when treating LGG-related epilepsy, it is more appropriate to focus on seizure outcome due to the risk of uncontrolled seizures developing into DRE, affecting patient development and quality of life. This review will focuses on the epidemiology, diagnosis, influencing factors, and surgical strategies for LGG-related epilepsy. We present the following article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-49/rc).
Methods
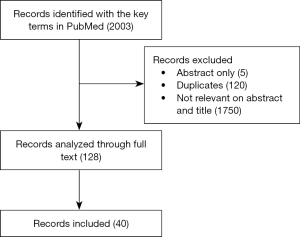
We searched all literatures published on PubMed, MEDLINE and EMBASE between January 1, 2001 and December 31, 2021 (Table 1, Figure 1). The searching terms “epilepsy, low grade glioma, management” were used to identify all full texts in English. The inclusion criteria are that the studies focused on the clinical management of epilepsy associated with low-grade glioma, including case report, retrospective study, prospective study and so on. The exclusion criteria are that the studies are not mentioned about management.
Table 1
| Items | Specification |
|---|---|
| Date of search | December 31, 2021 |
| Databases and other sources searched | PubMed, MEDLINE and EMBASE |
| Search terms used | Epilepsy, low grade glioma, management |
| Timeframe | January 1, 2001 to December 31, 2021 |
| Inclusion and exclusion criteria | The inclusion criteria are that the English studies focused on the clinical management of epilepsy associated with low-grade glioma, including case report, retrospective study, prospective study and so on. The exclusion criteria are that the studies are not mentioned about management |
| Selection process | The studies selection process was conducted by all authors, if the paper was not according with the inclusion criteria, after the discussion, it will be eliminated |
Epidemiology
According to the 2016 World Health Organization (WHO) Central Nerve System (CNS) tumor classification (6), glioma, the most common malignant brain tumor, can be categorized as low-grade glioma (LGG) or high-grade glioma (HGG). The incident rate of glioma-related epilepsy can range from 15% to 50% according to pathology and tumor location (7). While the mechanisms are still unclear, potential influencing factors include the growth pattern of the tumor, the presence of edema around the tumor, the microenvironment, and any particular gene alteration such as isocitrate dehydrogenase 1 (IDH1) gene mutation, or O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation (8). Some scholars believe that epilepsy and tumors have the same signaling pathway and molecular mechanism. However, the epileptogenicity of a tumor tends to stand in negative correlation to its malignancy. For example, pathologically benign dysembryoplastic neuroepithelial tumors (DNT) (9), one of the most common glioneuronal tumors, present with seizures almost 100% of the time. In general, LGG-related epilepsy may occur in up to 90% of LGG cases, in contrast to only 31% in the case of HGG. In a study carried out on 140 LGG patients, the epilepsy rate was 70–90% over the course of the disease. The incidence of epilepsy incidence also varies according to the type of LGG (10). For example, oligodendroglioma is more likely to induce seizures than astrocytoma (11), as confirmed by Chang et al. in a large cohort of patients. The reason for this may be related to the different tumor locations. Oligodendroglioma is commonly found in the cerebral cortex, which means they are more likely to induce epilepsy, while astrocytoma is usually found in the white matter region (12).
The variation in incidences of LGG-related epilepsy due to tumor location was also confirmed in a study on epilepsy caused by traumatic brain injury (13). It is generally believed that lesions on the motor cortex and temporal lobe are more likely to cause seizures. Duffau et al. reported that epilepsy presented in 39 out of 40 patients with LGG in the area surrounding the central sulcus (14). In the study reported by Chang et al., 86% of 111 patients with LGG of the temporal lobe presented with epilepsy. It should be noted that the majority of cases were drug-resistant epilepsy (DRE), which requires surgical treatment to render the patients seizure-free (15). Tumors located in deep midline structures or infratentorial spaces rarely cause epilepsy, although individual cases have been reported.
Medication
Regular anti-epileptic drug (AED) therapy is necessary when LGG-related epilepsy is diagnosed, irrespective of etiology. AED selection will depend on seizure type, patient age, underlying diseases, among other factors. Treatment is suitable for individualized and initial monotherapy with adequate dose and duration (16). AEDs are still recommended for a specified period. It is worth noting that AED selection must take drug-drug interactions into account to avoid effects such as hepatic enzyme induction. Meanwhile, chemotherapeutics can affect the metabolism and concentration of AEDs. Currently, levetiracetam (LEV) and valproic acid (VPA) are the AEDs recommended initially, as they tend to be well-tolerated and cause fewer side effects (17). Recent studies have revealed the combined effect of anti-cancer drugs with AEDs. VPA has been shown to inhibit glioma-genesis and prolong survival in patients with glioblastoma by suppressing histone deacetylase. In addition to its anti-epileptic effects, LEV has also been shown to synergize with temozolomide (TMZ) in the treatment of residual LGG after surgery (18,19). Other AEDs such as lacosamide (LCM), perampanel (PER), lamotrigine (LTG), pregabalin (PGB), zonisamide (ZON), and Brivaracetam (BRV) can be selected for add-on treatment (20). Preoperative prophylactic use of AEDs in LGG patients without preoperative seizures remains controversial. Most scholars believe there is no need for the prophylactic use of AEDs (21), while some suggest it is advisable for younger sufferers and those whose pathology involves the cortex or the temporal lobe.
Preoperative evaluation and surgical treatment
If LGG-related epilepsy is diagnosed, the surgical goal is maximal safe resection to render the patient seizure-free (22). Preoperative evaluation should be for “epilepsy surgery” rather than “tumor surgery” (23) and should include a detailed history, physical examination, MRI, ictal electroencephalogram (EEG), and psychological evaluation. The resection plan should include the entire tumor and any potential epileptogenic zones in the surrounding area. The preoperative evaluation If the seizure type, tumor location, or EEG is inconsistent, positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetoencephalography (MEG) can help identify the epileptogenic zones. Due to its invasiveness and cost, intracranial EEG is not routinely carried out in the preoperative evaluation of LGG (24).
As LGG is slow-growing, the focus of surgery is to perform a gross-total resection of the tumor, preserving patient functionality. Generally, the surgical spread border should include a 1–2 cm area surrounding the tumor in the nonfunctional cortex to ensure the complete resection of the epileptogenic zones (25). Electrocorticography (ECoG), neuronavigation, awake surgery, and “engraving surgery” can help identify the epileptogenic zones if necessary (26). Extended excision is not appropriate for LGG when located in the functional cortex or deep white matter, and intraoperative direct electrical stimulation for functional cortical or subcortical mapping is helpful.
Radiotherapy and chemotherapy on seizure-free patients
There are few reports on the effect of radiotherapy and chemotherapy for LGG on seizure-free patients after surgery. Because the LGG is slow-growing, there is still controversy surrounding the need for postoperative radiotherapy and chemotherapy (27). Some literature has suggested that such treatment may have a positive effect in reducing epilepsy. Rogers et al. found a 75% reduction of epilepsy after radiotherapy over a follow-up period of 8.2 years (28). The same result was also identified by Rossi et al. (29), who observed that stereotactic interstitial irradiation had a positive effect on epilepsy in patients with residual gliomas. The exact mechanism is still unclear but may be related to a reduction in tumor volume, damage to the tumor and surrounding epileptogenic zones, and changes in the microenvironment. It is worth noting that sometimes radiotherapy may cause immediate seizure onset in cases of acute edema, necrosis, or hemorrhage (30).
There have been several reports identifying improvements in seizure control with pharmacological anti-cancer treatment. Chemotherapy with temozolomide has been reported to reduce seizure frequency in 50–60% of patients with progressive LGG (31). The same result has been observed with a PCV (procarbazine, CCNU, vincristine) chemotherapy plan, although the detailed mechanism requires further study. In the interest of safety, residual LGG with epilepsy can be treated with radiotherapy or chemotherapy (32).
Prognosis
As LGG is slow-growing, the overall prognosis is excellent, with a median survival period of 5–10 years, which can extend up to 20 years. While the only factor for a favorable prognosis with LGG is gross total resection, irrespective of tumor type and location (33), the prognosis for LGG-related epilepsy has multiple factors (34). A gross total resection of LGG has been put forward as a positive predictor for seizure control. In Chang et al. (35), 89% of patients were seizure-free six months after a gross total resection, compared to only 57% after a biopsy or a subtotal tumor resection. The same conclusion was also reached by Packer et al., who reported 96% of patients to be seizure-free after total resection of the LGG (36). Surgical strategies also affect the prognosis of LGG-related epilepsy. The identification and complete removal of the epileptogenic zone will ensure the patient remains seizure-free in the long term (2,37). There is considerable literature suggesting that “epilepsy surgery” provides a higher seizure control rate than lesionectomy. Rossi et al. revealed a 66% seizure control rate after lesionectomy and a 79% control rate following “epilepsy surgery” in 48 cases of LGG-related epilepsy (38). Jooma et al. reported a different seizure control result for lateral temporal cortex LGG. Resection of the medial temporal lobe structure is an important factor for postoperative remission of epilepsy. Some scholars proposed a “dual pathology” phenomenon in lateral temporal cortex tumors commonly found in combination with hippocampal sclerosis (39). Otherwise, seizure type is the primary prognostic factor for LGG-related epilepsy. Partial seizures result in better control of epilepsy than general seizures (35). A shorter disease course in the preoperative period and younger patient age are also positive predictors for a long-term seizure-free outcome. Other factors include a lack of postsurgical seizures and a non-primary motor cortex glioma location.
Conclusions
LGG is the most common type of brain glioma, and epilepsy often presents as its only symptom. As the overall prognosis for LGG is good, given the clear differentiation between neural and glial cells, the importance of controlling seizures should be emphasized (40). Seizures that are not brought under control may develop into DRE, which will affect patient development, quality of life, and psychological well-being. If LGG-related epilepsy is diagnosed, any preoperative evaluation should be carried out for “epilepsy surgery” rather than “tumor surgery” (41). The goal of surgery is maximal safe resection to render the patient seizure-free, and gross total resection of LGG and the surrounding epileptogenic zones are the primary predictors for seizure control. Radiotherapy and chemotherapy both play a role in seizure control of with residual LGG (42). Given the favorable prognosis for LGG, perhaps controlling glioma-related epilepsy will become the primary therapeutic goal in the near future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ibrahim Qaddoumi, Anthony Liu and Chenchen Sun) for the series “Pediatric CNS Tumors in China” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-49/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-49/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-49/coif). The series “Pediatric CNS Tumors in China” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moshé SL, Perucca E, Ryvlin P, et al. Epilepsy: new advances. Lancet 2015;385:884-98. [Crossref] [PubMed]
- Blumcke I, Aronica E, Urbach H, et al. A neuropathology-based approach to epilepsy surgery in brain tumors and proposal for a new terminology use for long-term epilepsy-associated brain tumors. Acta Neuropathol 2014;128:39-54. [Crossref] [PubMed]
- Slegers RJ, Blumcke I. Low-grade developmental and epilepsy associated brain tumors: a critical update 2020. Acta Neuropathol Commun 2020;8:27. [Crossref] [PubMed]
- Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 2009;8:810-8. [Crossref] [PubMed]
- Glantz MJ, Batten J. Seizures and anti-epileptic drugs in neuro-oncology. Cancer Neurology In Clinical Practice: Springer; 2008. p. 33-46.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Dong H, Zhou XW, Wang X, et al. Complex role of connexin 43 in astrocytic tumors and possible promotion of glioma associated epileptic discharge Mol Med Rep 2017;16:7890-900. (Review). [Crossref] [PubMed]
- Takahashi Y, Nakamura H, Makino K, et al. Prognostic value of isocitrate dehydrogenase 1, O6-methylguanine-DNA methyltransferase promoter methylation, and 1p19q co-deletion in Japanese malignant glioma patients. World J Surg Oncol 2013;11:284. [Crossref] [PubMed]
- Luzzi S, Elia A, Del Maestro M, et al. Dysembryoplastic Neuroepithelial Tumors: What You Need to Know. World Neurosurg 2019;127:255-65. [Crossref] [PubMed]
- Ertürk Çetin Ö, İşler C, Uzan M, et al. Epilepsy-related brain tumors. Seizure 2017;44:93-7. [Crossref] [PubMed]
- Berntsson SG, Merrell RT, Amirian ES, et al. Glioma-related seizures in relation to histopathological subtypes: a report from the glioma international case-control study. J Neurol 2018;265:1432-42. [Crossref] [PubMed]
- Sánchez Fernández I, Loddenkemper T. Seizures caused by brain tumors in children. Seizure 2017;44:98-107. [Crossref] [PubMed]
- Vigil FA, Bozdemir E, Bugay V, et al. Prevention of brain damage after traumatic brain injury by pharmacological enhancement of KCNQ (Kv7, "M-type") K+ currents in neurons. J Cereb Blood Flow Metab 2020;40:1256-73. [Crossref] [PubMed]
- Duffau H, Capelle L, Denvil D, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg 2003;98:764-78. [Crossref] [PubMed]
- Dalic L, Cook MJ. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr Dis Treat 2016;12:2605-16. [Crossref] [PubMed]
- Kim KT, Kim DW, Yang KI, et al. Refining General Principles of Antiepileptic Drug Treatments for Epilepsy. J Clin Neurol 2020;16:383-9. [Crossref] [PubMed]
- Su LJ, Wang YL, Han T, et al. Antimyoclonic Effect of Levetiracetam and Clonazepam Combined Treatment on Myoclonic Epilepsy with Ragged-Red Fiber Syndrome with m.8344A>G Mutation. Chin Med J (Engl) 2018;131:2433-8. [Crossref] [PubMed]
- Bocangel DB, Finkelstein S, Schold SC, et al. Multifaceted resistance of gliomas to temozolomide. Clin Cancer Res 2002;8:2725-34. [PubMed]
- Siu A, Wind JJ, Iorgulescu JB, et al. Radiation necrosis following treatment of high grade glioma--a review of the literature and current understanding. Acta Neurochir (Wien) 2012;154:191-201; discussion 201. [Crossref] [PubMed]
- León Ruiz M, Rodríguez Sarasa ML, Sanjuán Rodríguez L, et al. Guidelines for seizure management in palliative care: Proposal for an updated clinical practice model based on a systematic literature review. Neurologia 2019;34:165-97. (Engl Ed). [PubMed]
- Director BJDDS. Oxford American handbook of clinical dentistry.: Oxford University Press, USA; 2007.
- Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol 2016;130:269-82. [Crossref] [PubMed]
- Takahashi A, Hong SC, Seo DW, et al. Frequent association of cortical dysplasia in dysembryoplastic neuroepithelial tumor treated by epilepsy surgery. Surg Neurol 2005;64:419-27. [Crossref] [PubMed]
- Morace R, Casciato S, Quarato PP, et al. Long-term seizure outcome in frontal lobe epilepsy surgery. Epilepsy Behav 2019;90:93-8. [Crossref] [PubMed]
- Lai D, Zhang X, Ma K, et al. Automated detection of high frequency oscillations in intracranial EEG using the combination of short-time energy and convolutional neural networks. IEEE Access 2019;7:82501-11.
- You G, Sha Z, Jiang T. Clinical Diagnosis and Perioperative Management of Glioma-Related Epilepsy. Front Oncol 2020;10:550353. [Crossref] [PubMed]
- Dhawan S, Patil CG, Chen C, et al. Early versus delayed postoperative radiotherapy for treatment of low-grade gliomas. Cochrane Database Syst Rev 2020;1:CD009229. [Crossref] [PubMed]
- Rogers G, Garside R, Mealing S, et al. Carmustine implants for the treatment of newly diagnosed high-grade gliomas: a cost-utility analysis. Pharmacoeconomics 2008;26:33-44. [Crossref] [PubMed]
- Rossi GF, Scerrati M, Roselli R. Epileptogenic cerebral low-grade tumors: effect of interstitial stereotactic irradiation on seizures. Appl Neurophysiol 1985;48:127-32. [PubMed]
- Gonzalez Castro LN, Milligan TA. Seizures in patients with cancer. Cancer 2020;126:1379-89. [Crossref] [PubMed]
- Haggiagi A, Avila EK. Seizure response to temozolomide chemotherapy in patients with WHO grade II oligodendroglioma: a single-institution descriptive study. Neurooncol Pract 2019;6:203-8. [Crossref] [PubMed]
- Walker AJ, Ruzevick J, Malayeri AA, et al. Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol 2014;10:1277-97. [Crossref] [PubMed]
- Ding X, Wang Z, Chen D, et al. The prognostic value of maximal surgical resection is attenuated in oligodendroglioma subgroups of adult diffuse glioma: a multicenter retrospective study. J Neurooncol 2018;140:591-603. [Crossref] [PubMed]
- Li F, Zhang Y, Wang N, et al. Evaluation of the Prognosis of Neuroglioma Based on Dynamic Magnetic Resonance Enhancement. World Neurosurg 2020;138:663-71. [Crossref] [PubMed]
- Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg 2008;108:227-35. [Crossref] [PubMed]
- Packer RJ, Sutton LN, Patel KM, et al. Seizure control following tumor surgery for childhood cortical low-grade gliomas. J Neurosurg 1994;80:998-1003. [Crossref] [PubMed]
- Brahimaj B, Greiner HM, Leach JL, et al. The surgical management of pediatric brain tumors causing epilepsy: consideration of the epileptogenic zone. Childs Nerv Syst 2014;30:1383-91. [Crossref] [PubMed]
- Rossi GF, Pompucci A, Colicchio G, et al. Factors of surgical outcome in tumoural epilepsy. Acta Neurochir (Wien) 1999;141:819-24. [Crossref] [PubMed]
- Jooma R, Yeh HS, Privitera MD, et al. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg 1995;83:231-6. [Crossref] [PubMed]
- Gallagher P, Leach JP, Grant R. Time to focus on brain tumor-related epilepsy trials. Neurooncol Pract 2014;1:123-33. [Crossref] [PubMed]
- Willemse RB, Hillebrand A, Ronner HE, et al. Magnetoencephalographic study of hand and foot sensorimotor organization in 325 consecutive patients evaluated for tumor or epilepsy surgery. Neuroimage Clin 2016;10:46-53. [Crossref] [PubMed]
- Tzikoulis V, Gkantaifi A, Alongi F, et al. Benign Intracranial Lesions - Radiotherapy: An Overview of Treatment Options, Indications and Therapeutic Results. Rev Recent Clin Trials 2020;15:93-121. [Crossref] [PubMed]
(English Language Editor: L. Roberts)
Cite this article as: Xu X, Li F. Clinical management of epilepsy associated with low-grade glioma and literature review. Pediatr Med 2022;5:41.