
The Swedish Neonatal Network for outcomes improvement
Background
At the end of the 1990’s, there was a call from the Swedish regional health care authorities, with financial support from the government, to create quality registers for benchmarking, quality improvement (QI) and research. In response to this initiative, a national working group of obstetricians and neonatologists was summoned to plan a perinatal register covering the reproductive chain of care, including the entities of maternal health care, fetal medicine, obstetrics, and neonatal care. The ambition was to cover all pregnancies and neonatal admissions in separate registers, interlinked on a shared platform with the unique personal identity number of the mother as a common key. The technical solution was developed in co-operation with an IT-provider (MedSciNet Ltd. Stockholm, Sweden) with manual records entered in web-forms and the data securely transferred through the Internet to the respective databases. As a result, the Swedish Neonatal Quality Register (SNQ) was established in 2001 (1).
The vision of Swedish neonatal services was that all newborn infants would receive the care they need, when they need it, that the experience was excellent for all families, and that neonatal care was executed by the highest quality and safety standards. To support this vision, the Swedish Neonatal Quality Register (SNQ) has had a mission to provide stake holders, professionals and the public with data and knowledge that would stimulate QI, clinical research and development in neonatal care.
The Swedish legislation concerning data acquisition for quality registers demands that the legal guardians must be informed about the data registered but consent for data collection is not needed. However, parents have a veto to opt out from having their infants’ information registered at any time. If, at a later stage the parents wish that their data should be deleted, this request is imperative and must be complied with. The written information (purpose, responsibilities, data recorded, usage, access and protection of the data, legal framework and patients’ rights) can be down-loaded without log-in (2).
In 2007, SwedROP—an affiliated register to SNQ—was launched by pediatric ophthalmologists. SwedROP collected data on all infants screened for retinopathy of prematurity (ROP), including ROP severity and treatment, and reported this data to SNQ. An expansion of SNQ occurred in 2012 when information on neonatal transports was added, including a structured assessment of the infant’s condition before and after transport (3), and a report of any adverse events during transport (4).
In 2015, a follow-up program was implemented by the Swedish Neonatal Society with records of neurosensory and neurodevelopmental status, and of cognitive function at 24 and 55 months of age in infants at significantly increased risk of compromised outcome. Since a main aim with all Swedish quality registers was to perform follow-up of health outcomes after hospital care, the information gathered at the 24 and 55 months’ follow-up examinations were reported to SNQ. The regulatory framework for this data collection was the same as for the neonatal data (2).
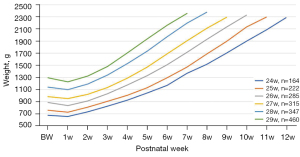
In 2016, the method for collecting data was changed from a paper form with a summary of data for the admission that was entered into a web-form usually at the end admission or later, to daily registration directly into a web-form which transferred the data immediately to the data base. This allowed feed back to the users with a minimum of delay and improved data quality considerably because when using repeated data extractions compared to one at the end of admission, the proportion of missing or incorrect data decreased significantly. Data could also be extracted to visualize the course of events over time for a defined group of infants or individually. Such applications could give a detailed picture of quality indicators, for example weight gain, oxygen consumption, type of respiratory support and skin to skin care (Figure 1). Furthermore, the timeline for occurrence of complications and treatments could also easily be extracted. However, the time-lag of one day was a limit for applying SNQ in clinical practice and for this purpose, most NICUs already have electronic patient data monitor systems.
Skilled nursing facilities, i.e., services put in place to provide neonatal care in the family’s home, has been growing in the last decades. This type of care prepares the family to detach safely from hospital care and shortens the length of hospital stay (5). Home care processes and outcomes (e.g., oxygen requirements at 36 weeks of postmenstrual age, growth, breast feeding) are recorded in SNQ on a daily basis. Currently, home care amounts to approximately 1/3 of the total amount of all neonatal admission days.
All hospitals have direct access to their own data, and benchmarking with deidentified, aggregated data on national or regional averages is possible. Comparisons between regions and units are done openly in reports and web-tools available on the homepage. Parents are also invited to send feed back to the health care provider. SNQ has a parental questionnaire which is sent by SMS to the parents’ mobile phone 14 days after discharge with the possibility to return the answer directly in the phone. Aggregated responses per neonatal unit are available online (https://www.medscinet.com/pnq/survey_PBI.aspx).
Structure and organization of networks
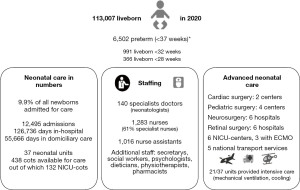
SNQ expanded gradually. Within the first three years (2001–2004) approximately 50% of all neonatal admissions were covered. By 2012, all admissions to all 37 neonatal units in the country—i.e., 6 academic centers providing full neonatal intensive care without a lower gestational age (GA) limit for admission (level III), 2 academic centers/units with a lower limit for neonatal intensive care of 25 weeks of GA (level III), 28 hospitals providing partial neonatal intensive care (level II), and 1 unit with neonatal care for near-term or term deliveries only (level I)—reported to SNQ, corresponding to approximately 11,000 infants (approximately 10% of all live-born infants) representing 14–15,000 annual admissions for neonatal care. SNQ has targeted care of preterm infants <35 weeks of GA (completeness 98–99%) and other newborn infants with major neonatal morbidities (1). Near-term to term infants with minor problems managed in the maternity units—such as phototherapy for moderate hyperbilirubinemia and glucose monitoring/extra oral feedings—could be but were not commonly reported to SNQ. Newborn infants coming from home to the hospital’s emergency room because of suspected infections or other unwellness have commonly been admitted to pediatric units not reporting to SNQ. In addition, infants with major malformations may have been referred from neonatal intensive care to surgical centers that do not report to SNQ. Seventy percent of all days in neonatal care registered in SNQ pertain to preterm infants with GA <37 weeks, as compared to the rate of preterm birth which has been around 6% of all live births (Figure 2).
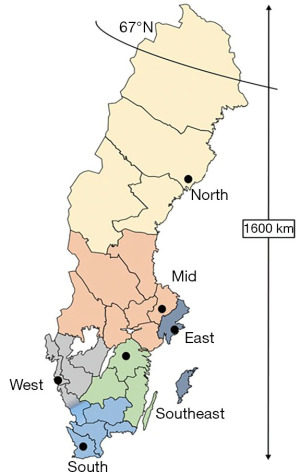
The organization of neonatal care follows 6 larger geographical regions, each region served by a university hospital with one or two level III neonatal intensive care units (Figure 3). Infants born at a level III hospital in one of the 6 regions could be transferred to another hospital in the region for a different (lower) level of care, once they had stabilized. In SNQ, 95% of the infants have had 1 or 2 admissions, whereas 5% of infants with the most complex morbidities (e.g., extremely preterm infants) have had 3 or more admissions before they could be discharged home for the first time. Given this organization in which several hospitals collaborate in networks around the same patient but at different levels of care, SNQ reports important outcomes such as survival for extremely preterm infants by these greater regions, and not per hospital (6).
Activities
Data entered into the SNQ has been found to be complete and valid, especially for preterm infants (1). SNQ is therefore used as a tool for benchmarking over time and between unit and regions.
Data are available at the open homepage (www.snq.se), in Annual Reports and at annual meetings for neonatal staff. There are also several ways of obtaining customized data from the register. Data are categorized in three dimensions: fitness for service or capacity (e.g., number of beds, staffing, competence, equipment), processes (interventions) and outcomes.
Capacity
The number of beds available for neonatal care is an indicator of capacity. In 2018, the number of neonatal beds in the six greater regions ranged from 2.5–6.9 per 1,000 live births (national average =4/1,000) and the number of neonatal intensive care beds ranged from 0.8–1.4 per 1,000 live births (national average =1/1,000).
Processes and outcomes
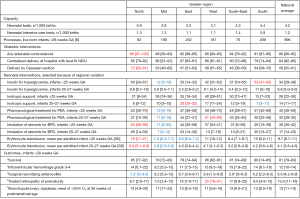
Benchmarking processes in extremely preterm infants born before 28 weeks of gestation include use of evidence-based practices such as antenatal corticosteroid treatment (national average in 2018–2020 =90%; range, 84–99%) and centralized delivery to a hospital with an in-house level III neonatal intensive care unit (national average =84%; range, 79–87%). Both these interventions have been associated with increased survival and decreased brain injuries in the most immature babies (7,8).
Interventions for extremely preterm infants with poor or unclear evidence (e.g., Cesarean section, pharmacological treatment for persistent ductus arteriosus and blood transfusions) sometimes show large regional variations (e.g., rates of inotrope use for hypotension, insulin for hyperglycemia and inhaled corticosteroids for bronchopulmonary dysplasia). Despite these differences in management, outcome measures in terms of survival and major neonatal morbidities show less variation between regions (Figure 4).
Benchmarking for moderately preterm and term infants, as well as nursing care, are also important parts of SNQ-activities. Procedural and outcome measures for these patient groups include but are not limited to rates of asphyxia and hypoxic-ischemic encephalopathy (and its treatment with hypothermia); admission rates after elective cesarean section; vascular access; neonatal pain; skin-to-skin care; skin injuries; infections and antibiotic treatment; neonatal hyperbilirubinemia and hypoglycemia.
Research activities
Research using SNQ is rapidly evolving and in 2020, 15 data-exports for research purposes were granted after approval of the Swedish Research Ethics Authority, and over 30 research projects is presently ongoing. So far, SNQ-data have been used in over 100 peer-reviewed publications (a complete list can be found at https://www.medscinet.com/PNQ/vetenskapliga-publikationer.aspx), sometimes after linkage to other registers.
QI is to provide new and better knowledge, change attitudes and implement more effective practices among providers of neonatal care. Formal training and education in QI is organized by regional quality centers, acting as support function for all quality registers and hospital departments in Sweden. SNQ has contributed to QI in the neonatal network by providing robust and valid data. A good example is the extremely preterm infants in Sweden study (EXPRESS) which started by building up knowledge (9-11), continued with data-driven changes in recommendations and national guidelines for management of extremely preterm births in Sweden, followed by a new register-based study on processes and outcome (12). Another example relates to SwedROP. Data from this SNQ-affiliated register have enabled continuous up-dates of national guidelines which have resulted in lower limits for ROP-screening (currently <30 weeks GA) and in a 20% reduction in the number of ROP-screening examinations in preterm infants without interfering with quality of care (13). SNQ also contributed to the forming of a strategy in the present national guideline which has been associated with a 25% reduction in early onset septicemia with group B streptococci (14). In addition, SNQ-data has been found to be useful to assess compliance to evidence-based recommendations, to quantify avoidable injuries in neonatal care, and to provide roadmaps for improved patient safety (15-17).
Besides targeting performance and benchmarking outcomes, SNQ has engaged in QI actions among participating centres resulting in 50% lower rates of late-onset neonatal septicemia in 2016–2020 (unpublished observation), and in improved neonatal nutrition in extremely preterm infants (18).
Current collaborations
SNQ collaborates with the Swedish Pregnancy Register (19). Both registers use the same IT-platform and collect and upload data on daily basis. Given permission from the Research Ethics Authority, the two registers can easily communicate via the personal identity number of the mother and a unique number for each pregnancy. Given daily reporting without delays and a much larger set of variables, these registers provide a higher data-resolution on care processes and timeliness than the Swedish Medical Birth Registry, which is a population register kept by the National Board of Health collecting diagnoses by International Classification of Diseases (ICD) codes only. The features of the Pregnancy and Neonatal registers have been useful for monitoring and on-line assessment of maternal and infant outcomes during the COVID-19 pandemic (20).
Other within country collaborations include the Swedish Neonatal Society (https://neo.barnlakarforeningen.se/), the Swedish Perinatal Society and parental organizations (https://prematurforbundet.se/). SNQ also provide stakeholders with statistics and data for governmental, authority and organizational reports on patient safety, potential for local and regional QIs, variations in availability of neonatal care, centralization of resources and dimensioning of perinatal care, outcomes for extremely preterm infants, ethics, withholding or withdrawing advanced life-support and parental experiences.
SNQ is part of the International Network for Evaluating Outcomes of Neonates, iNeo, coordinated from Toronto, Ontario, Canada (21). Besides benchmarking 11 regions in 10 countries on a number of different neonatal outcomes (22-25), the iNeo database has been instrumental for studies of rare exposures and rare outcomes in very preterm infants (26-28).
Another part of international collaboration has involved invitations to representatives of Neonatal Networks in other countries in the Nordic region (Norway, Finland and Denmark), other European countries (UK, Switzerland) and in North America (Canada, USA) as guest lecturers at SNQ-meetings.
Engagement activities for participating members
Each year, SNQ arranges national user meetings, workshops and webinars for education and to connect professionals in peri- and neonatal care, share experiences and learn from each other. These activities are often coordinated with patient and professional organizations. In 2019–2021, more than 20 local QI projects have been presented and discussed at these meetings.
Participating members use SNQ data for both clinical and research educational purposes. Several PhD- and master-theses have been based on SNQ-data and doctors and nurses can “get to know” data by reading the Annual SNQ report or extracting data of their choosing from the register.
Funding support
Besides federal population registers covering vital statistics and selected aspects of health care, there are more than 100 quality registers initiated and run by professionals working in the Swedish health care system. Basic funding of the national quality registers is provided by the Swedish Association of Local Authorities and Regions (SALAR) and the government. SALAR is an employers’ organization that represents and advocates for local government in Sweden. All of Sweden’s municipalities and regions are members of SALAR.
The amount of public support for the quality registers is based on standards and rating of each register by SALAR. SNQ has the highest rating (level 1 out of 4) by SALAR. Rating and funding are interrelated and evaluated annually.
In addition to funding from SALAR, participating hospitals contribute to SNQ with an annual fee. To strengthen finances, data exports for research have recently been charged for and meetings arranged by SNQ have been opened for commercial partnerships.
In return for their investments, SNQ provides SALAR and hospital administrations with data and reports. Recent developments with daily on-line data capture have increased the interest of departments heads in receiving customized, up-to-date reports on quality indicators, but also on production and workload.
Challenges—current and future
Currently, information on the mother, pregnancy and delivery is collected automatically for 92% of pregnant women in Sweden by an integration between the supplier of electronic medical records and the common IT-platform for the Swedish Pregnancy and Neonatal Quality Registers. However, daily reporting of neonatal care processes and outcomes, as well as follow-up data, are manually entered. A challenge for SNQ is to find ways to lessen the burden of manual data entry and to establish electronic transfer of data from medical records and monitor systems directly to the quality register.
The number of variables in SNQ exceeds 900. While a high granularity can be an asset for research purposes, the balance between having too many or too few indicators is more delicate when it comes to the workload of data entry and how to use the data for QI. The most important key performance indicators can be difficult to pin-point and preferences and needs vary between hospitals and over time. Standards for good performance are not easily set because many neonatal process indicators rest on a poor evidence base and show limited association to outcome. From a national and international perspective, large variability between centers in processes and outcomes seems particularly interesting to address, but it may also be challenging to interpret (22,23,29).
Funding of SNQ will remain an issue. Tax-funded support from the Government and SALAR covers only half of the present expenses and it will most likely not increase over time.
Future directions and plans
Currently, SNQ works in several directions. The two most important involve replacing manual data extraction with automatic data collecting from electronic medical health records and creating strategies for better and more interactive data outreach to the neonatal community. Other priorities include a better understanding of how to use the full power of daily outputs on processes and outcomes for QI, and to integrate parental experiences into neonatal practice. In addition, SNQ would like to inspire more and new research involving machine learning from big data-sets and register-based randomised controlled trials (RCTs), and strengthen international collaboration.
In conclusion, besides stimulating a continuously increasing number of clinical research projects, the data from SNQ has been found useful for QI by Swedish authorities, professional and stakeholder organizations, local hospital administrations, educators and teachers, as well as by the attending team in the neonatal unit.
Acknowledgments
Gunnar Sjörs, former Associate Director of the network is gratefully acknowledged for his devoted work for the development of SNQ. The authors also appreciate the contributions of all collaborators entering data into the network, with special thanks to Barbro Fossmo, Department of Pediatrics, Umeå University Hospital and Lena Swartling Schlinzig, Department of Neonatology, Karolinska University Hospital for dedicated support to network users.
Funding: This work was supported by The Swedish Association of Local Authorities and Regions (SALAR grant 079SNQ); by grants from a regional agreement on clinical research (ALF) between Region Stockholm and Karolinska Institutet (2020-0443; MN) and by the Childhood Foundation of the Swedish Order of Freemasons (MN).
Footnote
Provenance and Peer review: This article was commissioned by the Guest Editors (Shoo Lee and Prakesh Shah) for the series “Neonatal Networks for Outcomes Improvement: Evolution, Progress and Future” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-62/coif). The series “Neonatal Networks for Outcomes Improvement: Evolution, Progress and Future” was commissioned by the editorial office without any funding or sponsorship. The authors want to disclose that the data platform for SNQ is developed and maintained by the company MedSciNet AB/Ltd™, in May 2021 acquired by CSAM Health Group AS, Norway. MN reported funding from Region Stockholm and Karolinska Institutet (ALF 2020-0443); The Childhood Foundation of the Swedish Order of Freemasons and Chiesi Pharma AB; royalties from Studentlitteratur AB and Liber AB in Sweden; consultancy for Swedish Medical Journal and Swedish Patient Insurance; receipt of a lecture fee from Chiesi Pharma AB, and stocks in Eva & Mikael Norman AB. SH reported consultancy for the Swedish Neonatal Quality Register and shareholding in Essum AB. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Norman M, Källén K, Wahlström E, et al. The Swedish Neonatal Quality Register - contents, completeness and validity. Acta Paediatr 2019;108:1411-8. [Crossref] [PubMed]
- Information to patients and their relativers regarding the processing of personal data by the Swedish national quality registers [cited 2021 Sept 30]. Available online: https://www.medscinet.com/PNQ/uploads/website/translations/English%20-%20engelska.pdf
- Lee SK, Zupancic JA, Pendray M, et al. Transport risk index of physiologic stability: a practical system for assessing infant transport care. J Pediatr 2001;139:220-6. [Crossref] [PubMed]
- van den Berg J, Olsson L, Svensson A, et al. Adverse events during air and ground neonatal transport: 13 years' experience from a neonatal transport team in Northern Sweden. J Matern Fetal Neonatal Med 2015;28:1231-7. [Crossref] [PubMed]
- Ortenstrand A, Winbladh B, Nordström G, et al. Early discharge of preterm infants followed by domiciliary nursing care: parents' anxiety, assessment of infant health and breastfeeding. Acta Paediatr 2001;90:1190-5. [Crossref] [PubMed]
- Rysavy MA, Marlow N, Doyle LW, et al. Reporting Outcomes of Extremely Preterm Births. Pediatrics 2016;138:e20160689. [Crossref] [PubMed]
- Helenius K, Longford N, Lehtonen L, et al. Association of early postnatal transfer and birth outside a tertiary hospital with mortality and severe brain injury in extremely preterm infants: observational cohort study with propensity score matching. BMJ 2019;367:l5678. [Crossref] [PubMed]
- Norman M, Piedvache A, Børch K, et al. Association of Short Antenatal Corticosteroid Administration-to-Birth Intervals With Survival and Morbidity Among Very Preterm Infants: Results From the EPICE Cohort. JAMA Pediatr 2017;171:678-86. [Crossref] [PubMed]
- EXPRESS Group. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 2009;301:2225-33. [Crossref] [PubMed]
- EXPRESS Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS). Acta Paediatr 2010;99:978-92. [Crossref] [PubMed]
- Serenius F, Källén K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013;309:1810-20. [Crossref] [PubMed]
- Norman M, Hallberg B, Abrahamsson T, et al. Association Between Year of Birth and 1-Year Survival Among Extremely Preterm Infants in Sweden During 2004-2007 and 2014-2016. JAMA 2019;321:1188-99. [Crossref] [PubMed]
- Holmström G, Hellström A, Gränse L, et al. New modifications of Swedish ROP guidelines based on 10-year data from the SWEDROP register. Br J Ophthalmol 2020;104:943-9. [Crossref] [PubMed]
- Håkansson S, Lilja M, Jacobsson B, et al. Reduced incidence of neonatal early-onset group B streptococcal infection after promulgation of guidelines for risk-based intrapartum antibiotic prophylaxis in Sweden: analysis of a national population-based cohort. Acta Obstet Gynecol Scand 2017;96:1475-83. [Crossref] [PubMed]
- Alkén J, Håkansson S, Ekéus C, et al. Rates of Extreme Neonatal Hyperbilirubinemia and Kernicterus in Children and Adherence to National Guidelines for Screening, Diagnosis, and Treatment in Sweden. JAMA Netw Open 2019;2:e190858. [Crossref] [PubMed]
- Norman M, Hellström A, Hallberg B, et al. Prevalence of Severe Visual Disability Among Preterm Children With Retinopathy of Prematurity and Association With Adherence to Best Practice Guidelines. JAMA Netw Open 2019;2:e186801. [Crossref] [PubMed]
- Challis P, Nydert P, Håkansson S, et al. Association of Adherence to Surfactant Best Practice Uses With Clinical Outcomes Among Neonates in Sweden. JAMA Netw Open 2021;4:e217269. [Crossref] [PubMed]
- Westin V, Klevebro S, Domellöf M, et al. Improved nutrition for extremely preterm infants - A population based observational study. Clin Nutr ESPEN 2018;23:245-51. [Crossref] [PubMed]
- Stephansson O, Petersson K, Björk C, et al. The Swedish Pregnancy Register - for quality of care improvement and research. Acta Obstet Gynecol Scand 2018;97:466-76. [Crossref] [PubMed]
- Norman M, Navér L, Söderling J, et al. Association of Maternal SARS-CoV-2 Infection in Pregnancy With Neonatal Outcomes. JAMA 2021;325:2076-86. [Crossref] [PubMed]
- Shah PS, Lui K, Reichman B, et al. The International Network for Evaluating Outcomes (iNeo) of neonates: evolution, progress and opportunities. Transl Pediatr 2019;8:170-81. [Crossref] [PubMed]
- Helenius K, Sjörs G, Shah PS, et al. Survival in Very Preterm Infants: An International Comparison of 10 National Neonatal Networks. Pediatrics 2017;140:e20171264. [Crossref] [PubMed]
- Lui K, Vento M, Modi N, et al. Inter-center variability in neonatal outcomes of preterm infants: A longitudinal evaluation of 298 neonatal units in 11 countries. Semin Fetal Neonatal Med 2021;26:101196. [Crossref] [PubMed]
- Lehtonen L, Lee SK, Kusuda S, et al. Family Rooms in Neonatal Intensive Care Units and Neonatal Outcomes: An International Survey and Linked Cohort Study. J Pediatr 2020;226:112-117.e4. [Crossref] [PubMed]
- Lui K, Lee SK, Kusuda S, et al. Trends in Outcomes for Neonates Born Very Preterm and Very Low Birth Weight in 11 High-Income Countries. J Pediatr 2019;215:32-40.e14. [Crossref] [PubMed]
- Shah PS, Kusuda S, Håkansson S, et al. Neonatal Outcomes of Very Preterm or Very Low Birth Weight Triplets. Pediatrics 2018;142:e20181938. [Crossref] [PubMed]
- Persson M, Shah PS, Rusconi F, et al. Association of Maternal Diabetes With Neonatal Outcomes of Very Preterm and Very Low-Birth-Weight Infants: An International Cohort Study. JAMA Pediatr 2018;172:867-75. [Crossref] [PubMed]
- Norman M, Håkansson S, Kusuda S, et al. Neonatal Outcomes in Very Preterm Infants With Severe Congenital Heart Defects: An International Cohort Study. J Am Heart Assoc 2020;9:e015369. [Crossref] [PubMed]
- Zeitlin J, Maier RF, Cuttini M, et al. Cohort Profile: Effective Perinatal Intensive Care in Europe (EPICE) very preterm birth cohort. Int J Epidemiol 2020;49:372-86. [Crossref] [PubMed]
Cite this article as: Norman M, Håkansson S; for The SNQ Collaboration. The Swedish Neonatal Network for outcomes improvement. Pediatr Med 2023;6:9.

