
Clinical characteristics and prognosis of pediatric medulloblastoma: a cohort study of 40 patients at Children’s Hospital of Soochow University
Introduction
Medulloblastoma (MB) is a common malignant tumor that presents during childhood, typically 6–8 years of age, and accounts for approximately 20% of all central nervous system (CNS) tumors in children (1,2). When treated with a combination of surgery, postoperative radiotherapy, and postoperative chemotherapy, the long-term survival rate of MB is 60–80% (3). Risk stratification criteria for MB had historically been based on age, the presence of metastasis, tumor resection extent, and histological subtype-related clinicopathological variables. In some cases, it has also been associated with individual genetic abnormalities, such as MYC and MYCN amplification. Advancements in gene transcriptome analysis have allowed insights gained from whole genome and gene transcription analysis to be transformed into clinical practice. In 2010, an international group of experts applied transcription profiling to reach a consensus defining the four main subgroups of MB. Each subgroup has a unique transcription profile, specific somatic DNA variations, and distinct clinical outcomes. The 2015 World Health Organization (WHO) Consensus Meeting recognized the importance of these biological subgroups, and introduced the following genetically-defined MB classification into the revised 2016 WHO Classification of Tumors of the Central Nervous System: wingless-activated (WNT-activated) group, sonic hedgehog-activated (SHH-activated) group, Group 3, and Group 4. A unique treatment plan for each subgroup was formulated, according to the molecular classification, in order to improve the clinical outcomes. In this study the authors analyzed the clinical data of children with MB treated at Children’s Hospital of Soochow University between November 2011 and October 2020, and the clinical characteristics and prognosis of MB. We present the following article in accordance with the STROBE reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-59/rc).
Methods
Clinical data
Inclusion criteria: (I) age of onset and initial diagnosis <14 years old; (II) surgery performed, with postoperative pathology to confirm the diagnosis as MB; (III) complete follow-up data; (IV) exclude patients who died within 1 week post-operation (such cases may be related to surgical errors and belong to short-term deaths during hospitalization). There were 222 cases of CNS tumors at Children’s Hospital of Soochow University between November 2011 and October 2020, with 44 cases of MB (44/222, 20.0%). Four of these cases died within one week post-operation, leaving 40 cases of pediatric MB for inclusion in this study. The basic clinical characteristics of these patients were analyzed, including: age, gender, whether or not a complete resection was performed, histological classification, molecular classification, clinical stage, whether or not the patient received radiotherapy/chemotherapy, and whether or not recurrence/progression was observed. All patients were followed up, either by electronic medical record or by phone; the endpoint for follow-up was 31 December 2020. The follow-up included magnetic resonance imaging (MRI) evaluation and whether or not patients had experienced neuro-related clinical manifestations. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Children’s Hospital of Soochow University. All guardians of patients gave oral consent and signed informed consent forms.
Study methods
Histological classification and molecular classification are based on the 2016 WHO Classification of Tumors of the Central Nervous System (4). Standard histological preparations (hematoxylin and eosin) were used to assess general architectural and cytological features, including nodule formation, differentiation along neuronal (neurocytic/ganglionic) and astrocytic lines, and large cell or anaplastic phenotypes. Reticulin preparations were used to evaluate desmoplasia. Internodular desmoplasia was required for a diagnosis of desmoplastic/nodular (D/N) MB. Molecular detection used fluorescence in-situ hybridization and immunohistochemistry (IHC).
Definition of the degrees of MB metastasis
Non-metastatic stage
M0 means that the tumor is limited to the area in which it grew and there is no evidence of metastasis.
Metastatic stage
M1 means that only cerebrospinal fluid tumor cells tested positive; M2 means that gross nodular seeding is present in the cerebellar subarachnoid space and/or lateral ventricle or third ventricle; M3 means that gross nodular seeding is present in the spinal subarachnoid space; M4 means that metastasis has occurred outside the cerebrospinal axis.
Risk stratification
Age ≥3 years old
Standard risk: total/near total tumor resection (residual tumor ≤1.5 cm2) and no metastasis (M0). High risk: subtotal resection (residual tumor >1.5 cm2) or with metastasis.
Age <3 years old
Standard risk: total/near total tumor resection (residual tumor ≤1.5 cm2), no metastasis (M0), and the histological subtype is desmoplastic/nodular.
High risk: all cases that don’t fall into the standard risk group are high-risk cases.
Treatment
Surgical intervention for all children sought to remove as much of the tumor as possible and restore cerebrospinal fluid flow.
Patients ≥3 years old at the time of diagnosis were dosed with radiation according to their risk group. For standard-risk cases, the posterior fossa or local tumor bed radiation dose was 54–55 Gy, and the whole brain and spinal cord radiation dose was usually 23.4 Gy. High-risk patients received 54–55 Gy radiotherapy for the posterior fossa or local tumor bed, and 36 Gy radiotherapy for the whole brain and whole spinal cord. During the radiotherapy period, vincristine (VCR) was given per each week by intravenous injection: a 1.5 mg/m2 dose was administered 6–8 times in total. Adjuvant chemotherapy was started 4 weeks after completion of radiotherapy. The chemotherapy regimen consisted of 8 × 6-week cycles of semustine, cisplatin (DDP), and VCR.
For patients <3 years old at the time of diagnosis, those with standard risk did not receive radiotherapy after surgery; for high-risk patients, radiotherapy or local radiotherapy was postponed until patients reached 3 years of age. Adjuvant chemotherapy was started 2–4 weeks after surgery, consisting of 12 cycles of cyclophosphamide (CTX), VCR/high dose methotrexate/carboplatin (CBP), etoposide (VP-16) alternate chemotherapy, administered over 2-week intervals.
Statistical analysis
The overall survival (OS) is defined as the period (in months) from the date of surgery to the date of the last follow-up or death; the survival rate during this period is called the overall survival rate. The event-free survival (EFS) is the time (in months) from surgical resection to tumor recurrence, metastasis, or tumor progression; the survival rate during this period is called the EFS rate. Recurrences were confirmed by imaging examinations (computed tomography, MRI etc.). SPSS 23.0 was used to perform statistical analysis on the data: median for non-normal distribution data; the Kaplan-Meier method for the OS curve; the log-rank test was used to compare the survival rates; and Cox regression analysis was used to analyze factors affecting the survival time. A P value <0.05 is considered statistically significant.
Results
General information
A total of 40 MB patients were analyzed in this study, including 20 males and 20 females. The age of tumor onset ranges from 5 months to 163 months, with a median age of 81 months. Complete resection was performed in 26 cases (26/40, 65%), while 14 cases (14/40, 35%) underwent subtotal resection. Metastasis had already occurred in 8 cases at the time of initial diagnosis. 32 cases were classic type (32/40, 80%), 6 were D/N type (6/40, 15%), and 2 were anaplastic type (2/40, 5%). Molecular classification of 19 cases by IHC found 1 case belonging to the WNT-activated subgroup (1/19, 5.3%), 6 cases belonging to the SHH-activated subgroup (6/19, 31.6%), 7 cases belonging to the Group 3 subgroup (7/19, 36.8%), and 5 cases belonging to the Group 4 subgroup (5/19, 26.3%). Excluding those cases that attended other hospitals due to economic or personal factors post-surgery (8 patients returned to their local hospitals for medical insurance and government funds, while another 3 patients went to other hospitals at their parents’ discretion), 29 patients received combined radiotherapy and chemotherapy treatment. The follow-up time ranged from 1 month to 102 months, with a median follow-up time of 25 months (1–102 months). At the time of the last follow-up, 20 patients were alive and 9 patients had died (all due to tumor recurrence). Overall, the 3-year OS rate was (64.3±10.4)% and the 3-year EFS rate was (61.8±10.3)%. The 3-year EFS rate of the metastatic group was (20.0±17.9)%, and in the non-metastatic group it was (74.5±10.1)% (P<0.05). The 3-year EFS rate for patients <3 years old was (25.0±21.7)%, while for patients ≥3 years old it was (67.9±11.1)% (P<0.05). The 3-year EFS rate of the D/N group was higher than that of the other two groups, and the difference was statistically significant (P<0.05). The 3-year EFS rate of the radiotherapy group and the non-radiotherapy group (Figure 1) were (70.7±11.2)% and (20.0±17.9)%, respectively, and the difference was statistically significant (P<0.05).
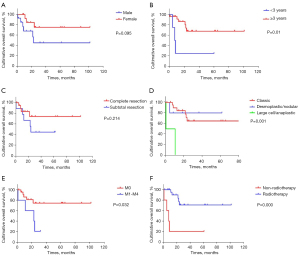
Univariate and multivariate Cox regression to analyze variables affecting the prognosis of children with MB
Clinical characteristics of patients were considered, including: age, gender, histological classification, clinical stage, and extent of resection. It was found that the OS of patients ≥3 years of age at the time of initial diagnosis with D/N subtype and no metastasis was significantly better than that of patients <3 years of age at the time of initial diagnosis with other histological types and with metastasis. The difference was statistically significant, with a P value <0.05. The difference for other variables was not statistically significant (Table 1 and Figure 1). EFS and OS were affected by the same variables.
Table 1
| Variables | Number | OS, % | P | EFS, % | P | |
|---|---|---|---|---|---|---|
| Gender | Male | 14 | 43.4±15.8 | 0.114 | 45.4±15.8 | 0.095 |
| Female | 15 | 83.9±10.4 | 75.2±12.6 | |||
| Age | <3 years | 4 | 25.0±21.7 | 0.036 | 25.0±21.7 | 0.01 |
| ≥3 years | 25 | 72.4±10.8 | 67.9±11.1 | |||
| Residual tumor | Complete resection | 20 | 79.1±11.1 | 0.245 | 74.0±11.8 | 0.214 |
| Subtotal resection | 9 | 55.6±16.6 | 44.4±16.6 | |||
| Clinical stage | M0 | 24 | 78.1±9.8 | 0.034 | 74.5±10.1 | 0.032 |
| M1-M4 | 5 | 40.0±21.9 | 20.0±17.9 | |||
| Histological subtype | Classic | 22 | 68.7±11.8 | 0.000 | 65.0±11.7 | 0.001 |
| Desmoplastic/nodular | 5 | 80.0±17.9 | 80.0±17.9 | |||
| Large cell/anaplastic | 2 | 0 | 0 | |||
| Radiotherapy | Radiotherapy | 24 | 75.4±10.8 | 0.002 | 70.7±11.2 | 0.000 |
| Non-radiotherapy | 5 | 20.0±17.9 | 20.0±17.9 |
OS, overall survival; EFS, event-free survival.
Multivariate Cox regression analysis (Table 2) showed that age <3 years old at the time of initial diagnosis and tumor metastasis were independent risk factors leading to a poor prognosis in pediatric MB (P<0.05).
Table 2
| Factors | B | SE | Wald χ2 | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Clinical stage | 3.580 | 1.642 | 4.756 | 1.4–896.0 | 0.029 |
| Age | –3.629 | 1.229 | 8.716 | 0.002–0.295 | 0.02 |
| Residual tumor | –1.885 | 1.445 | 1.701 | 0.01–2.58 | 0.192 |
| Gender | –1.152 | 0.909 | 1.605 | 0.05–1.88 | 0.316 |
| Histological subtype | 0.607 | 0.351 | 2.995 | 0.92–3.65 | 0.084 |
| Radiotherapy | 0.402 | 0.908 | 0.196 | 0.25–8.86 | 0.658 |
Discussion
The main clinical symptoms of pediatric MB are headache, vomiting, ataxia, and nystagmus, with an annual incidence rate of 0.2–0.58/100,000 (5). Although current treatment protocols achieve an OS of about 70% for childhood MB, more than 1/3 of patients die within 5 years of diagnosis, with a median survival time for relapsed/refractory MB of less than 1 year (6). MB accounts for 20% of the CNS tumors identified at Children’s Hospital of Soochow University in the past decade. Except for one patient who died 15 months after recurrence, all patients considered in this study who relapsed or exhibited disease progression died within 1 year.
In 2010, a meta-analysis found that age, histological type, pre-operative dissemination of tumor, and post-operative residual tumor size are independent factors that affect the prognosis of children with MB, and are relevant for risk stratification (7). Children who were more than 3 years old at the time of initial diagnosis, with no metastasis and who received post-operative radiotherapy, had a greater OS than those who were younger than 3 years old at the time of initial diagnosis, with metastases (M2–M4 stages) and who did not receive radiotherapy. These findings are consistent with previous research reports (7-9). The results of the factor analysis presented here show that clinical stage and age at initial diagnosis are independent factors affecting prognosis. Although the univariate analysis of the patients found that the extent of tumor resection had no statistically significant effect on OS, the OS of children in the complete tumor resection group was greater than that of the partial resection group. The difference may be more significant if the sample size is enlarged.
Chemotherapy and radiotherapy are crucial in improving the outcome of brain tumors. Post-operative radiotherapy is currently recognized as an effective method for MB treatment. Radiotherapy usually starts within 30 days after surgery, and delayed radiotherapy is associated with reduced survival rates (10-12). Earlier studies showed that for high-risk pediatric MB patients, adjuvant chemotherapy improved the disease-free survival rate (DFS) (13), as compared to patients who only received radiotherapy. But another large study reported in 2006 showed that EFS was unaffected by chemotherapy regimen (14). A total of 379 standard-risk MB patients received craniocerebral radiotherapy (23.4 Gy) and posterior fossa radiotherapy (55.8 Gy), and were randomly assigned to one of two adjuvant chemotherapy regimens: lomustine, DDP, and VCR; or CTX, DDP, and VCR. The 5-year EFS and OS of the 379 patients were (81±2.1)% and (86±9)%, respectively.
In this study, excluding all post-operative treatment cases who went to other hospitals for economic or personal reasons, a total of 29 children received standard radiotherapy and chemotherapy after surgery. The OS of children with post-operative radiotherapy was longer than for those without radiotherapy, and the difference was statistically significant. Since the patients’ brains are in the developmental phase, especially for those children under 3 years old, radiotherapy can cause adverse reactions such as growth and development abnormalities, endocrine abnormalities, and cognitive dysfunction. The major treatment for patients younger than 3 years old is chemotherapy, together with delayed radiotherapy or local radiotherapy (15).
MBs are divided into 4 subtypes based on their histological characteristics: classic type, D/N type, extensive nodularity type, and large cell/anaplastic type. In previous studies the D/N type was found to have the best outcome, and the large cell/anaplastic type showed the worst outcome (16,17). Consistent with reported results, the OS of the D/N type was higher than that of the other two groups, and the prognosis of the anaplastic type is relatively poor in this study. Due to the small sample size of this study, there is a lack of cases with extensive nodularity.
In the past decade, with the development of integrative genomics, our knowledge and understanding of the biology of MBs has greatly expanded. At the molecular level, WHO classified MBs into four subgroups: WNT-activated group, SHH-activated group1, Group 3 and Group 4. There are some overlaps between histological and molecular subtypes: D/N type, hyperplasia nodular type, and extensive nodularity type are almost exclusively SHH-activated MB; large cell/anaplastic type are enriched in TP53-mutated SHH-activated group or high-risk Group 3; in WNT-activated group and Group 4, the role of identifying large cells/anaplasia is unclear (3). The WNT-activated group is the least common molecular group, accounting for only 10% of all MBs; tumors activate the WNT signaling pathway, which mainly occurs in elderly patients, equally distributed between both genders and rarely associated with metastasis, with a 5-year OS of 95%. The SHH-activated group accounts for about 30% of all MBs, with a bimodal age distribution. The incidence increases in infants and children under 5 years old, and then increases again in adolescents and adults over 16 years old, with a 5-year OS of 75%. Group 3 accounts for about 25% of all cases, and has the worst outcome with a 5-year OS of 50%. Finally Group 4, known as the most common subgroup, accounts for about 35% of all MBs, and has a moderate prognosis with a 5-year OS of 75% (18-23).
Specific treatment methods for molecular subgroups are also being continuously studied. The integrity of the vascular endothelium and blood-brain barrier are often destroyed in WNT-activated MB, increasing the permeability of systemic chemotherapy and leading to a positive prognosis (24). The destruction of the blood-brain barrier is thought to be due to secretion of the diffusible WNT antagonists WNT inhibitory factor 1 (WIF1) and Dickkopf-related protein 1 (25). Clinical trials (NCT01878617 and NCT02212574) currently take advantage of these insights in the hope that the WNT-activated subgroup can be effectively treated with less chemotherapy and/or radiotherapy. About 80% of SHH-activated MB patients carry patched-1 (PTCH1) or smoothened (SMO) mutations. Inhibition of SMO provides a targeted treatment approach for SHH-activated MB patients. A clinical trial (NCT03734913) explored the effect of SMO protein inhibitor ZSP1602 on patients with advanced MB. Limitations in understanding the mechanism of MB occurrence in Group 3 and Group 4 hinders the development of targeted therapy strategies. In Group 3 MB, preclinical studies have focused on inhibiting phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) signaling pathways, evaluating the synergistic activity between histone deacetylase inhibitors and PI3K inhibitors, evaluating the efficacy of cyclin-dependent kinase (CDK), protein kinase, DNA-activated, catalytic subunit (PRKDC) or bromodomain and extra-terminal (BET) bromodomain inhibitors, and testing the effectiveness of anti-angiogenesis treatments (26).
In this study, only 19 patients were subject to molecular subtyping tests, and the proportions of WNT-activated group and SHH-activated group MBs were similar to the results of previous studies. However, the proportions of Group 3 and Group 4 were quite different, which may be related to the small sample size. There was only one patient in the WNT-activated group, and although the tumor had metastasized at the time of initial diagnosis (M3), the patient was still in a disease-free survival state after receiving post-operative standard radiotherapy and chemotherapy with 3 years of follow-up treatment. Given the relatively small number of children who were subject to molecular subtyping tests and standard treatment, and considering the large deviation of the results, statistical analysis was not performed. Further analysis will be performed when there are more patients enrolled.
In spite of the fact that MBs account for a significant proportion of childhood CNS tumors, the prognosis for MBs in the high risk group is still unsatisfactory, especially in children with tumor recurrence. Research on prognostic factors is helpful for conducting risk stratification, designing treatment plans, and improving patient quality of life.
Acknowledgments
The authors would like to acknowledge Ms. Chenchen Sun for her translation of the manuscript from Chinese to English.
Funding: This study was supported by the National Natural Science Foundation of China (NSFC) 81802499, Jiangsu project (CXTDA2017014, BE2019672), Suzhou project (SS201809, GSWS2020039, KJXW2018016), and National Clinical Research Center for Hematological Disorders (2020ZKPB02).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ibrahim Qaddoumi, Anthony Liu and Chenchen Sun) for the series “Pediatric CNS Tumors in China” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-59/rc
Data Sharing Statement: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-59/dss
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-59/coif). The series “Pediatric CNS Tumors in China” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Children’s Hospital of Soochow University. All guardians of patients gave oral consent and signed informed consent forms.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
1Including tumor protein 53 (TP53)-mutated SHH and TP53 wild-type SHH
References
- Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 2012;19:1541-4. [Crossref] [PubMed]
- Medulloblastoma. Nat Rev Dis Primers 2019;5:12. [Crossref] [PubMed]
- Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol 2016;131:821-31. [Crossref] [PubMed]
- Wen PY, Huse JT. 2016 World Health Organization Classification of Central Nervous System Tumors. Continuum (Minneap Minn) 2017;23:1531-47. [Crossref] [PubMed]
- Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev 2014;23:2716-36. [Crossref] [PubMed]
- Pui CH, Gajjar AJ, Kane JR, et al. Challenging issues in pediatric oncology. Nat Rev Clin Oncol 2011;8:540-9. [Crossref] [PubMed]
- Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol 2010;28:4961-8. [Crossref] [PubMed]
- Nalita N, Ratanalert S, Kanjanapradit K, et al. Survival and Prognostic Factors in Pediatric Patients with Medulloblastoma in Southern Thailand. J Pediatr Neurosci 2018;13:150-7. [Crossref] [PubMed]
- Wang C, Yuan XJ, Jiang MW, et al. Clinical characteristics and abandonment and outcome of treatment in 67 Chinese children with medulloblastoma. J Neurosurg Pediatr 2016;17:49-56. [Crossref] [PubMed]
- Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017;547:311-7. [Crossref] [PubMed]
- Schwalbe EC, Lindsey JC, Nakjang S, et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol 2017;18:958-71. [Crossref] [PubMed]
- Juraschka K, Taylor MD. Medulloblastoma in the age of molecular subgroups: a review. J Neurosurg Pediatr 2019;24:353-63. [Crossref] [PubMed]
- Packer RJ, Siegel KR, Sutton LN, et al. Efficacy of adjuvant chemotherapy for patients with poor-risk medulloblastoma: a preliminary report. Ann Neurol 1988;24:503-8. [Crossref] [PubMed]
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 2006;24:4202-8. [Crossref] [PubMed]
- Pulsifer MB, Duncanson H, Grieco J, et al. Cognitive and Adaptive Outcomes After Proton Radiation for Pediatric Patients With Brain Tumors. Int J Radiat Oncol Biol Phys 2018;102:391-8. [Crossref] [PubMed]
- Archer TC, Mahoney EL, Pomeroy SL. Medulloblastoma: Molecular Classification-Based Personal Therapeutics. Neurotherapeutics 2017;14:265-73. [Crossref] [PubMed]
- von Bueren AO, Kortmann RD, von Hoff K, et al. Treatment of Children and Adolescents With Metastatic Medulloblastoma and Prognostic Relevance of Clinical and Biologic Parameters. J Clin Oncol 2016;34:4151-60. [Crossref] [PubMed]
- Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012;123:465-72. [Crossref] [PubMed]
- Ramaswamy V, Remke M, Shih D, et al. Duration of the pre-diagnostic interval in medulloblastoma is subgroup dependent. Pediatr Blood Cancer 2014;61:1190-4. [Crossref] [PubMed]
- Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 2011;29:1424-30. [Crossref] [PubMed]
- Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 2011;29:1408-14. [Crossref] [PubMed]
- Khatua S, Song A, Citla Sridhar D, et al. Childhood Medulloblastoma: Current Therapies, Emerging Molecular Landscape and Newer Therapeutic Insights. Curr Neuropharmacol 2018;16:1045-58. [Crossref] [PubMed]
- Kijima N, Kanemura Y. Molecular Classification of Medulloblastoma. Neurol Med Chir (Tokyo) 2016;56:687-97. [Crossref] [PubMed]
- Phoenix TN, Patmore DM, Boop S, et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016;29:508-22. [Crossref] [PubMed]
- Daneman R, Agalliu D, Zhou L, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A 2009;106:641-6. [Crossref] [PubMed]
- Menyhárt O, Giangaspero F, Győrffy B. Molecular markers and potential therapeutic targets in non-WNT/non-SHH (group 3 and group 4) medulloblastomas. J Hematol Oncol 2019;12:29. [Crossref] [PubMed]
(English Language Editor: E. Davies)
Cite this article as: Du W, Lu Y, Yang F, Lu Q, Li Z, Lu J, Hu S. Clinical characteristics and prognosis of pediatric medulloblastoma: a cohort study of 40 patients at Children’s Hospital of Soochow University. Pediatr Med 2022;5:12.

