
Clinical features and prognostic analysis of children with medulloblastoma in a single center in China
Introduction
Medulloblastoma (MB) is the most common malignant pediatric brain tumor, accounting for nearly 20% of all central nervous system malignancies in patients younger than 18 years. Its incidence peaks at the ages of 3–4 and 8–9 years. MB accounts for 60% of childhood intracranial embryonal tumors (1-3). Standard-of-care therapy for MB includes surgical resection of the tumor, chemotherapy, and craniospinal irradiation (CSI), which are usually performed in children older than 3 years. Treatment outcome is affected by several factors, such as age, extent of resection, the receipt of chemotherapy or radiotherapy, and histomorphological or molecular features. After standard comprehensive treatment such as surgery, radiotherapy, or chemotherapy, the 5-year event-free survival (EFS) rate is 70–80% in the standard-risk group vs. 60% in the high-risk group (2,4). In this study, we summarized the clinical characteristics and prognosis of children diagnosed with MB in the Medical Oncology Department of Beijing Children’s Hospital between 2012 and 2020. We present the following article in accordance with the STROBE reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-34/rc).
Methods
Patients and eligibility
We enrolled children with newly diagnosed, pathologically confirmed, postoperative MB. They received chemotherapy in the Medical Oncology Department of Beijing Children’s Hospital from January 2012 to December 2020. Thirty-one patients with MB were enrolled. The study was approved by the Ethics Committee of Beijing Children’s Hospital (No. 2020-Z-012) and was conducted in accordance with the tenets of the Helsinki Declaration (as revised in 2013). Individual consent for this retrospective analysis was waived.
Staging and risk groups
Metastasis was assessed using the Chang staging system. M0 was defined as the absence of metastasis according to both imaging and cerebrospinal fluid (CSF) analyses. M1 was defined as the presence of tumor cells only in CSF without evidence of metastasis on imaging. Gross nodular seeding in the cerebellum, cerebral subarachnoid space, or third or fourth ventricles was defined as M2, and gross nodular seeding in the spinal subarachnoid space was defined as M3. M4 was defined as the presence of extraneuroaxial metastasis (5). Experienced radiologists were responsible for the assessment of residual tumor on magnetic resonance imaging (MRI). CSF pathology analysis was performed to determine whether there was CSF metastasis.
Patients were stratified postoperatively by age and clinical and histological criteria into a standard-risk or high-risk group. The diagnostic criteria for the standard-risk group for patients older than 3 years were M0 cancer and residual tumors smaller than 1.5 cm² on postoperative MRI with any histology. The criteria for high-risk patients older than 3 years included subtotal resection with residual tumors of 1.5 cm² or larger in size, evidence of metastatic diseases (M1–M4), and large cell/anaplastic histology. Among children younger than or equal to 3 years, those with M0 lesions, postoperative residual tumors smaller than 1.5 cm2, and histologically desmoplastic nodular or MB with extensive nodularity were categorized into the standard-risk group, whereas the remaining patients were classified into the high-risk group.
Surgery
All patients underwent surgical resection of the primary tumor to the maximal extent, and tumor tissue was obtained for diagnosis and biological examination. The extent of resection was evaluated by postoperative MRI and the surgeon’s assessment. MRI of the brain was performed within 72 hours after surgery to assess the presence of residual tumor. Gross total resection indicated that no tumor mass was visible after surgery as confirmed by MRI. Gross total or near-total resection was defined as residual tumor smaller than or equal to 1.5 cm2, and subtotal or partial resection was defined as residual tumor larger than 1.5 cm2. Patients with obstruction of CSF flow underwent the placement of an external ventricular catheter or ventriculoperitoneal shunt to resolve the problem of obstruction.
Chemotherapy
Children with MB received different therapeutic regimens based on their ages. For patients younger than 3 years, chemotherapy (12 cycles of 2 weeks each) was started 2–4 weeks after surgery. The protocol included cyclophosphamide (800 mg/m² intravenously for 3 days) and vincristine (1.5 mg/m² intravenously for 1 day) in cycles 1, 5, and 9. Intravenous methotrexate (5 g/m² on day 1) and vincristine (1.5 mg/m² on day 1) were provided in cycles 2, 3, 6, 7, 10, and 11. Carboplatin (200 mg/m²) and etoposide (150 mg/m²) were administered for 3 days in cycles 4, 8, and 12. For patients older than 3 years, radiotherapy was initiated approximately 4 weeks after surgery. Chemotherapy (8 cycles of 21 days each) was started 4 weeks after radiotherapy. Patients in all risk groups received vincristine (1.5 mg/m² intravenously on days 1, 8, and 15), cisplatin (75 mg/m² for 1 day), and cyclophosphamide (750 mg/m2 for 2 days) in cycles 1, 2, 3, 4, 7, and 8. The patients intravenously received carboplatin (200 mg/m²) and etoposide (150 mg/m²) on days 1–3 in cycles 5 and 6. Autologous hematopoietic stem cell rescue after high-dose chemotherapy was suggested for high risk patients regardless of age. The high-dose chemotherapy included thiotepa (300 mg/m² for 3 days), carboplatin (500 mg/m² for 3 days) and etoposide (250 mg/m² for 3 days).
Radiation therapy
Radiotherapy was administered 4 weeks after surgery in patients older than 3 years. Radiotherapy comprised focal radiotherapy for the tumor bed and craniospinal radiotherapy over 6 weeks. For patients with standard-risk MB, the radiotherapy dose to the tumor bed was 54–55 Gy, and the reduced dose of CSI was 23.4 Gy; meanwhile, high-risk patients received 36 Gy of CSI and 54–55 Gy to the local tumor bed.
Evaluation and follow-up
MRI of the brain and spine and CSF cytology were required for disease staging. MRI of the spine was performed before or 2 weeks after surgery to determine the presence of metastatic lesions. Most children could not undergo lumbar puncture to obtain CSF before surgery because of the presence of intracranial hypertension. CSF cytology was performed during operation or 2 weeks after surgery via lumbar puncture. Patients were evaluated at regular intervals. The assessment included MRI, CSF cytology, and an examination of therapy-related toxicity. Liver, kidney, and heart function; and auditory response were monitored. In patients with CSF metastasis, CSF cytology was performed until 2 consecutive negative results were obtained. Disease evaluation was performed every 2 months during chemotherapy, every 3 months during the first year after treatment, every 6 months in years 2–3 after treatment, and annually until 5 years after treatment. The date of last follow-up in this study was February 28, 2021.
Statistical analysis
EFS was calculated from the day of diagnosis to an event (first relapse or progression, second malignancy, or death of any cause) or to the date of last follow-up if no event occurred. Overall survival (OS) was defined as the interval from enrollment to death or the last follow-up. Statistical analyses were performed using SPSS for Windows, version 19.0 (IBM Corp., Armonk, NY, USA). OS and EFS were estimated using the Kaplan-Meier method. Nonnormally distributed data are expressed as the median (range), and Wilcoxon’s signed-rank test was used for group comparisons. Qualitative data are presented as percentages (%), and χ2 test was used to assess differences between groups for categorical variables. A P value <0.05 indicated statistical significance.
Results
Patients’ clinical data
Thirty-one patients (17 boys, 14 girls) with newly diagnosed MB were enrolled in this study and retrospectively analyzed. The median patient age at the time of diagnosis was 73 months (range, 11–168 months). Metastasis was present in 15 patients at time of diagnosis, including 1, 1, 9, and 4 patients with M1, M2, M3, and both M2 and M3 metastasis, respectively. There was no patient with extraneuroaxial metastasis. The clinical characteristics of the 31 patients are shown in Table 1.
Table 1
| Characteristics of patients | N (%) |
|---|---|
| Sex | |
| Male | 17 (54.8) |
| Female | 14 (45.2) |
| Histology subtypes | |
| Classic | 11 (35.5) |
| DN | 6 (19.4) |
| MBEN | 1 (3.2) |
| LCA | 1 (3.2) |
| Including 2 histology subgroups | 6 (19.4) |
| Unknown | 6 (19.4) |
| Molecular subtypes | |
| WNT | 4 (12.9) |
| SHH | 8 (25.8) |
| Group 3 | 5 (16.1) |
| Group 4 | 6 (19.4) |
| Unknown | 8 (25.8) |
| Primary tumor location | |
| Vermis | 17 (54.8) |
| Fourth ventricle | 9 (29.0) |
| Brainstem | 4 (12.9) |
| Cerebellar hemisphere | 1 (3.2) |
| Extent of resection | |
| Gross total or near-total resection | 26 (83.9) |
| Subtotal or partial resection | 5 (16.1) |
| Ventriculoperitoneal shunt | |
| Yes | 14 (45.2) |
| No | 17 (54.8) |
| CMS after surgery | |
| Yes | 16 (51.6) |
| No | 15 (48.4) |
| M Stage | |
| M1+ | 15 (48.4) |
| M0 | 16 (51.6) |
| Risk subgroups | |
| Standard risk | 11 (35.5) |
| High risk | 20 (64.5) |
LCA, large cell, anaplastic; DN, desmoplastic/nodular; MBEN, medulloblastoma with extensive nodularity; SHH, sonic hedgehog activated; WNT, wingless activated; CMS, cerebellar mutism syndrome.
Sixteen patients (51.6%) developed cerebellar mutism syndrome (CMS) after surgery. There was no significant difference in age, sex, tumor location, longest tumor diameter, extent of resection, molecular subtype, or pathological subtype between patients with and without CMS (all P values >0.05; Table 2).
Table 2
| Characteristics of patients | Number of patients with CMS (n=16) | Number of patients without CMS (n=15) |
|---|---|---|
| Median age at diagnosis, months (range) | 63 [23–97] | 82 [11–168] |
| Sex, n (%) | ||
| Male | 9 (56.3) | 8 (53.3) |
| Female | 7 (43.7) | 7 (46.7) |
| Primary tumor location, n (%) | ||
| Vermis | 9 (56.3) | 8 (53.3) |
| Fourth ventricle | 4 (25.0) | 5 (33.3) |
| Brainstem | 3 (18.8) | 1 (6.7) |
| Cerebellar hemisphere | 0 | 1 (6.7) |
| Median longest tumor diameter, cm (range) | 4.4 (1.9–6.0) | 4.6 (2.4–8.3) |
| Extent of resection, n (%) | ||
| Gross total or near-total resection | 13 (81.3) | 13 (86.7) |
| Subtotal or partial resection | 3 (18.8) | 2 (13.3) |
| Histology subtypes, n (%) | ||
| Classic | 8 (50.0) | 3 (20.0) |
| DN | 1 (6.3) | 5 (33.3) |
| MBEN | 1 (6.3) | 0 |
| LCA | 1 (6.3) | 0 |
| Including two histology subgroups | 2 (12.5) | 4 (26.7) |
| Unknown | 3 (18.8) | 3 (20.0) |
| Molecular subtypes, n (%) | ||
| WNT | 2 (12.5) | 2 (13.3) |
| SHH | 3 (18.8) | 5 (33.3) |
| Group 3 | 4 (25.0) | 1 (6.7) |
| Group 4 | 4 (25.0) | 2 (13.3) |
| Unknown | 3 (18.8) | 5 (33.3) |
CMS, cerebellar mutism syndrome; LCA, large cell/anaplastic; DN, desmoplastic/nodular; MBEN, medulloblastoma with extensive nodularity; SHH, sonic hedgehog activated; WNT, wingless activated.
Treatment
All patients underwent surgery. The extent of resection was gross total or near-total resection in 26 patients (83.9%) vs. subtotal or partial resection in 5 patients (16.1%); 9 patients received radiotherapy before chemotherapy, 16 patients received chemotherapy first after surgery, 5 patients received only chemotherapy, and 1 patient did not receive radiotherapy or chemotherapy after resection of primary tumor. These data are presented in Figure 1.
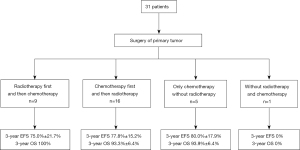
Twenty-five children received radiotherapy of the local tumor bed and CSI. The median radiotherapy dose was 54 Gy (range, 49.2–60 Gy) at the primary site and 36 Gy (range, 23.4–36 Gy) for CSI. The median time between surgery and radiotherapy was 71.5 days (range, 29–457 days). The median time between surgery and radiotherapy for the 9 patients who received radiotherapy first was 36 days (range, 29–62 days). The median time between surgery and chemotherapy among the 16 patients who received chemotherapy first was 31.5 days (range, 16–59 days).
Thirty patients received chemotherapy in this study. The median number of chemotherapy cycles was 8 (range, 1–14 cycles). Sixteen patients developed CMS after surgery. All patients with CMS received chemotherapy before irradiation. The median number of chemotherapy courses before radiotherapy was 2 (range, 1–14 courses). The median time interval between surgery and irradiation was 94 days (range, 29–457 days).
Survival analysis
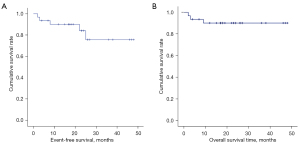
The median follow-up time was 19 months (range, 2–48 months). Three patients experienced tumor relapse or progression at 5, 8, and 25 months after surgery, respectively. Three patients died, with one dying due to relapse of MB at 9 months after diagnosis and 2 dying due to hydrocephalus and cerebral hernia at 2 and 3 months after surgery, respectively. The 3-year EFS and OS rates were 75.7%±10.6% and 90.1%±5.4%, respectively (Figure 2).
The 3-year EFS rate was 75.3%±13.7% in the 25 patients who received radiotherapy, vs. 66.7%±19.2% in the 6 patients who did not receive radiotherapy (χ2=1.386; P=0.239). The 3-year OS rate was 95.8%±4.1% in the 25 patients who received radiotherapy, compared with 66.7%±19.2% in the 6 patients who did not receive radiotherapy (χ2=5.935; P=0.015). Twenty-five children received radiotherapy after surgery. The 3-year EFS rate was 75.0%±21.7% in patients who received radiation first, vs. 77.8%±15.2% in those treated with chemotherapy first (χ2=0.273; P=0.601). The 3-year OS rate was 100% in patients treated with radiation first, compared with 93.3%±6.4% in patients treated with chemotherapy first (χ2=0.600; P=0.439).
Prognostic analysis
Univariate survival analysis identified age <3 years and the omission of radiotherapy as poor prognostic factors (both P values <0.05; Table 3).
Table 3
| Characteristics of Patients | Number of patients, n (%) | 3-year EFS (%) | P value | 3-year OS (%) | P value |
|---|---|---|---|---|---|
| Sex | 0.693 | 0.674 | |||
| Male | 17 (54.8) | 87.8±8.1 | 87.8±8.1 | ||
| Female | 14 (45.2) | 68.8±15.8 | 92.9±6.9 | ||
| Age at diagnosis | 0.054 | 0.046 | |||
| <3 years | 7 (22.6) | 53.6±20.1 | 71.4±17.1 | ||
| ≥3 years | 24 (77.4) | 81.8±13.2 | 95.5±4.4 | ||
| Histology subtypes | 0.297 | 0.757 | |||
| Classic | 11 (35.5) | 64.6±17.5 | 80.8±12.2 | ||
| DN | 6 (19.4) | 100 | 100 | ||
| MBEN | 1 (3.2) | 100 | 100 | ||
| LCA | 1 (3.2) | 100 | 100 | ||
| Including two histology subgroups | 6 (19.4) | 100 | 100 | ||
| Unknown | 6 (19.4) | 83.3±15.2 | 83.3±15.2 | ||
| Molecular subtypes | 0.377 | 0.422 | |||
| WNT | 4 (12.9) | 100 | 100 | ||
| SHH | 8 (25.8) | 85.7±13.2 | 85.7±13.2 | ||
| Group 3 | 5 (16.1) | 100 | 100 | ||
| Group 4 | 6 (19.4) | 66.7±27.2 | 100 | ||
| Unknown | 8 (25.8) | 50±22.8 | 75±15.3 | ||
| Extent of resection | 0.854 | 0.455 | |||
| Gross total or near-total resection | 26 (83.9) | 74.6±12.4 | 92.3±5.2 | ||
| Subtotal or partial resection | 5 (16.1) | 80.0±17.9 | 80±17.9 | ||
| Ventriculoperitoneal shunt | 0.488 | 0.674 | |||
| Yes | 14 (45.2) | 59.7±20 | 92.9±6.9 | ||
| No | 17 (54.8) | 87.8±8.1 | 87.8±8.1 | ||
| CMS after surgery | 0.091 | 0.588 | |||
| Yes | 16 (51.6) | 46.2±22.0 | 86.5±8.9 | ||
| No | 15 (48.4) | 93.3±6.4 | 93.3±6.4 | ||
| M Stage | 0.718 | 0.537 | |||
| M1+ | 15 (48.4) | 75.4±12.8 | 86.2±9.1 | ||
| M0 | 16 (51.6) | 70.3±20.8 | 93.8±6.1 | ||
| Risk subgroups | 0.104 | 0.19 | |||
| Standard risk | 11 (35.5) | 100 | 100 | ||
| High risk | 20 (64.5) | 65.3±13.7 | 84.7±8.1 | ||
| Radiotherapy | 0.239 | 0.015 | |||
| Yes | 25 (80.6) | 75.3±13.7 | 95.8±4.1 | ||
| No | 6 (19.4) | 66.7±19.2 | 66.7±19.2 |
EFS, event-free survival; OS, overall survival; LCA, large cell/anaplastic; DN, desmoplastic/nodular; MBEN, medulloblastoma with extensive nodularity; SHH, sonic hedgehog activated; WNT, wingless activated; CMS, cerebellar mutism syndrome.
Discussion
Conventional treatment, which involves a combination of surgery, radiotherapy, and chemotherapy, can achieve long-term OS in 60–80% of children with MB (1,2).
The histopathological classification of MB comprises the classic, desmoplastic/nodular, MB with extensive nodularity, and large cell/anaplastic subtypes, with prognosis varying across these subtypes.von Bueren et al. reported that patients younger than 4 years with desmoplastic/nodular MB had better 5-year EFS and OS rates (90% and 100%, respectively) than did patients with the classic subtype (30% and 68%, respectively; P<0.001 for EFS; P<0.008 for OS) and/or large cell/anaplastic subtype (33%) (6). In our study, 7 patients were younger than 3 years at the time of diagnosis including 3 children with desmoplastic/nodular variants who received chemotherapy alone. These three patients were followed up without event for 28, 46, and 48 months, respectively. Moreover, 50% of patients with classic MB experienced relapse after chemotherapy and then received radiotherapy after the age of 3 years. Another child with classic MB died of hydrocephalus and hernia 3 months after surgery. One patient with extensive nodularity MB received radiotherapy after chemotherapy at 3 years of age. He exhibited survival without disease progression for 17 months. Li et al. reported that the 5-year OS in MB patients with desmoplastic/nodular subtype was significantly better than that with classical subtype and with large cell/anaplastic subtype (72% vs. 51% vs. 0%; P=0.0032) (7). Sustained tumor control can be achieved using chemotherapy alone after surgery in young children with desmoplastic/nodular variants. However, children at least 3 years old with non-desmoplastic/non-nodular variants should receive local radiotherapy after chemotherapy (6).
According to the World Health Organization Classification of 2016, MB includes 4 biological subgroups: wingless (WNT)-activated, sonic hedgehog (SHH)–activated, group 3, and group 4. WNT-activated MB rarely metastasizes, and it has a favorable prognosis, including a long-term survival rate exceeding 95%. Group 3 is considered the most aggressive subtype, as indicated by its 5-year OS rate of less than 60%, particularly in infants and children with metastatic disease and/or MYC amplification (1,2). In our study, 4 patients with WNT-activated MB had standard-risk disease without metastases. The 3-year EFS and OS rates in these patients were both 100%. Five patients with group 3 MB also had good prognoses in our study, as indicated by 3-year EFS and OS rates of 100%. Further analysis illustrated that the median age of these patients was 82 months, which was older than the median age of the other subgroups. In addition, they had no metastasis at the time of diagnosis or MYC amplification. These factors influenced the good prognosis of these patients. The prognosis for patients with group 4 MB was intermediate. The prognosis of SHH-activated MB was dependent on age, tumor histology, the presence of metastasis, and genotype. Patients with SHH-activated MB with metastasis and/or MYC amplification were considered to have high-risk disease (survival rate of 50–75%).
In TP53-mutated tumors, the survival rate has been reported to be less than 50% (1,2). In our study, the group 4 subgroup included 6 patients, 5 of whom had cerebrospinal metastases at the time of diagnosis, indicating high-risk disease and explaining the 3-year EFS rate of 66.7% in this group. A further 8 patients had SHH-activated MB in our study, including 7 patients with cerebrospinal metastasis at the time of diagnosis, 1 patient with MYCN-amplified MB, and 1 patient with concurrent TP53 mutation and MYCN amplification.
Children younger than 3 years with MB had a worse long-term survival rate than did the older children. The 2-year OS rate ranged from 46% to 70% in younger children in prior research, whereas the 5-year OS rate ranged from 13% to 60% (3,4). Declining cognitive function and sensory function is concerning to clinicians during and after radiotherapy in younger children. Thus, radiotherapy was omitted to avoid long-term side effects (8). Johnston et al. reviewed the outcome of children with MB younger than 36 months old at diagnosis in Canada. In total, about 30% of the patients (n=20) who received chemotherapy alone were alive at the time of the survey compared to about 74% of those treated with both chemotherapy and radiotherapy (3). In our study, 28.6% of children younger than 3 years received radiotherapy, and their 3-year OS rate was 71.4% compared to the 95.5% rate in older children (P=0.046). Meanwhile, 95.8% of children older than 3 years received radiotherapy. However, this study only included 7 children younger than 3 years, 2 of whom died of hydrocephalus and hernia after surgery. Additionally, 2 patients who received chemotherapy and radiotherapy and 3 patients who received chemotherapy alone were alive at the time of the survey. We need to examine additional cases to assess the efficacy and safety of radiotherapy in young children. In the COG ACNS1221 clinical trial, the 2-year progression-free survival (PFS) rate was only 52% in patients younger than 4 years of age with MB who received the regimen of conventional chemotherapy without intraventricular methotrexate injection (9). In our study, the young children received intravenous high-dose methotrexate without intrathecal or intraventricular methotrexate. The 3-year EFS in children younger than 3 years of age was 53.6% and that in children older than 3 years of age was.81.8% (P=0.054). The 3-year OS was 71.4% and 95.5% (P=0.046), respectively. This can be partly explained by the fact that the young children did not receive radiotherapy, and perhaps another reason was that there was no intraventricular methotrexate injection. A prospective clinical trial with a large amount of data should be designed to compare the survival of intrathecal or intravascular methotrexate in young children.
CMS is a common complication following the resection of posterior fossa tumors, occurring in 24% of children with MB. CMS generally emerges within 2 to 3 days after surgery concomitantly with speech deficits, emotional lability, and hypotonia (10,11). In our study, the rate of postoperative CMS reached 51.6%, which was much higher than that reported previously. It was possible that children with dysphasia from direct surgical nerve or brainstem injury were incorrectly diagnosed with CMS. In addition, several patients with CMS who could not receive radiotherapy first came to our center for chemotherapy first, while patients without CMS received radiotherapy first at other hospitals. This might also have resulted in a high proportion of CMS in our data. The midline location of the cerebellar vermis in most patients with CMS is considered to be pathogenesis of the syndrome (10,11). In a prior study, brainstem invasion by the tumor was a risk factor for CMS regardless of the tumor stage, median age at diagnosis, sex, extent of tumor resection, or postoperative complications (11). In our study, there was no significant correlation of CMS with age, sex, the extent of tumor resection, or tumor size. The location of tumors was the vermis in 56.3% of patients with CMS vs. 53.3% of patients without CMS. The tumor was located in the brain stem in 3 patients with CMS and 1 patient without CMS. The number of cases was insufficient to determine the correlation between brainstem invasion and CMS. Patients with CMS were younger, and they had larger tumors. WNT-activated, group 3 and group 4 variants were found to be independently associated with a higher risk of CMS compared to the SHH-activated subtype (10). In our study, SHH-activated MB was more common in patients without CMS (33.3%), but not significantly so (P=0.487). Traditionally, patients with MB should start radiation therapy within 4 to 5 weeks of surgery, and delayed postoperative radiotherapy might decrease the long-term prognosis. The HIT 2000 Trial report showed that a time interval between surgery and irradiation of less than 48 days could yield a better PFS of 80.4% when compared with the interval of more than 49 days (PFS 64.6%; P=0.052) (12). However, Chin et al. showed that the time to radiotherapy of more than 5 weeks but within 90 days of surgery did not adversely impact the 5-year OS rate (P=0.563) (13). In our study, all patients with CMS received chemotherapy before irradiation because CMS patients could not be coordinated with radiotherapy after surgery. The median time interval between surgery and irradiation was 94 days. The 3-year EFS rate and OS rate were 75.0% vs. 77.8% (P=0.601) and 100% vs. 93.3% (P=0.439) in patients who received radiation first and chemotherapy first, respectively. As the number of cases in our study was too small to draw a definitive conclusion, further large sample studies should be conducted in the future.
Surgery plays an important role in the management of MB. The degree of surgical resection is one of the most critical prognostic factors for long-term OS. However, the prognostic importance of the extent of resection has not been well defined. Previous studies identified gross total resection of the tumor as a strongly positive predictor of survival (2). Thompson et al. systematically reviewed the PubMed database to clarify the correlation between the extent of resection and prognosis in patients with MB. Sixteen articles reported there to be a significant association between the extent of tumor resection and survival, 20 articles found no association, and 14 articles reported mixed results (14). Johnston et al. found that incomplete resection in younger children did not affect OS when metastatic disease was present. There was no significant difference in survival between patients with <90% resection and >90% resection (3). Thompson et al. also analyzed the prognostic value of the extent of resection in 787 patients with MB. They found a PFS benefit for gross total resection compared with subtotal resection when the molecular subgroup was considered. However, there was no definitive benefit of gross total resection vs. near-total resection (15). In studies from different Chinese hospitals, Jiang and Li from reported that the 5-year EFS and 5-year OS in patients with MB who underwent gross total resection were significantly higher than those of subtotal resection (7,16). In our study, there was no significant difference in prognosis between gross total/near-total resection and subtotal resection. Five patients underwent subtotal resection, and the correlation of subtotal resection with molecular subtypes was uncertain. However, the neurological morbidity rate was high when overly aggressive surgical resection was performed. The neurologic morbidity rate among children undergoing MB resection is as high as 44% after gross total resection (2). The extent of tumor resection should be determined with the aim of reducing the complications of surgery.
Pathological subtype is a prognostic factor in patients younger than 3 years with MB. The prognosis of children with desmoplastic/nodular MB is good, and the prognosis varies by molecular subtype. In the era of precision medicine, molecular subtype and advantages and disadvantages of radiotherapy in young children should be considered for the selection of treatment.
The main limitation of our study lies in the small number cases acquired from a single center in China, which make it difficult to draw definitive conclusions from the data. More multicenter clinical studies should be carried out to obtain a greater amount of data from Chinese MB patients. The short follow-up time was also one of the shortcomings of this paper, and thus extending the follow-up time would aid in finding more precise prognostic information of patients with MB.
Acknowledgments
The authors would like to thank the patients and their parents for their support during this study. We also thank the study staff of Beijing Children’s Hospital, Beijing Tiantan Hospital, and Peking Union Medical College Hospital for their contributions to this article.
Funding: The study was funded by Beijing Hospital Authority Ascent Plan (No. DFL20191201).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ibrahim Qaddoumi, Anthony Liu and Chenchen Sun) for the series “Pediatric CNS Tumors in China” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at: https://pm.amegroups.com/article/view/10.21037/pm-21-34/rc
Data Sharing Statement: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-34/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-34/coif). The series “Pediatric CNS Tumors in China” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Beijing Children’s Hospital (No. 2020-Z-012). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khatua S, Song A, Citla Sridhar D, et al. Childhood Medulloblastoma: Current Therapies, Emerging Molecular Landscape and Newer Therapeutic Insights. Curr Neuropharmacol 2018;16:1045-58. [Crossref] [PubMed]
- Northcott PA, Robinson GW, Kratz CP, et al. Medulloblastoma. Nat Rev Dis Primers 2019;5:11. [Crossref] [PubMed]
- Johnston DL, Keene D, Bartels U, et al. Medulloblastoma in children under the age of three years: a retrospective Canadian review. J Neurooncol 2009;94:51-6. [Crossref] [PubMed]
- Robinson GW, Rudneva VA, Buchhalter I, et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol 2018;19:768-84. [Crossref] [PubMed]
- Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology 1969;93:1351-9. [Crossref] [PubMed]
- von Bueren AO, von Hoff K, Pietsch T, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol 2011;13:669-79. [Crossref] [PubMed]
- Li T, Zhou X, Hai LL, et al. Correlation analysis of histopathology type and prognosis of posterior fossa medulloblastoma in children. Chinese Journal of Practical Nervous Diseases 2019;22:1173-8.
- Padovani L, Horan G, Ajithkumar T. Radiotherapy Advances in Paediatric Medulloblastoma Treatment. Clin Oncol (R Coll Radiol) 2019;31:171-81. [Crossref] [PubMed]
- Lafay-Cousin L, Bouffet E, Strother D, et al. Phase II Study of Nonmetastatic Desmoplastic Medulloblastoma in Children Younger Than 4 Years of Age: A Report of the Children's Oncology Group (ACNS1221). J Clin Oncol 2020;38:223-31. [Crossref] [PubMed]
- Jabarkheel R, Amayiri N, Yecies D, et al. Molecular correlates of cerebellar mutism syndrome in medulloblastoma. Neuro Oncol 2020;22:290-7. [PubMed]
- Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg 2006;105:444-51. [PubMed]
- Dietzsch S, Placzek F, Pietschmann K, et al. Evaluation of Prognostic Factors and Role of Participation in a Randomized Trial or a Prospective Registry in Pediatric and Adolescent Nonmetastatic Medulloblastoma - A Report From the HIT 2000 Trial. Adv Radiat Oncol 2020;5:1158-69. [Crossref] [PubMed]
- Chin AL, Moding EJ, Donaldson SS, et al. Survival impact of postoperative radiotherapy timing in pediatric and adolescent medulloblastoma. Neuro Oncol 2018;20:1133-41. [Crossref] [PubMed]
- Thompson EM, Bramall A, Herndon JE 2nd, et al. The clinical importance of medulloblastoma extent of resection: a systematic review. J Neurooncol 2018;139:523-39. [Crossref] [PubMed]
- Thompson EM, Hielscher T, Bouffet E, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol 2016;17:484-95. [Crossref] [PubMed]
- Jiang T, Wang JM, Du J, et al. Analysis of clinical prognosis and risk factors for children with medulloblastoma. Chin J Neurosurg 2016;32:338-43.
Cite this article as: Su Y, Zhu S, Qin J, Ge M, Ji Y, Gong J, Yu T, Fu L, Liu Z, Ma X. Clinical features and prognostic analysis of children with medulloblastoma in a single center in China. Pediatr Med 2022;5:13.

