Laboratory testing for the diagnosis of neonatal and pediatric immune neutropenias: a narrative review
Introduction
Neutropenia is a relatively common condition in children and, based on the absolute neutrophil count (ANC), is classified as severe (<500/µL), moderate (500–1,000/µL), or mild (1,000–1,500/µL). In the Western population, the lower limit of neutrophils is 1,500/µL from one year of age, while it is 1,000/µL in children from 2 months to one year (1). These reference values may not be suitable for other ethnic groups, especially for people of African heritage or Middle Eastern groups, where the normal limits of ANC are lower (2).
In the pediatric population, neutropenia is often immune-mediated. Antibodies, directed against human neutrophil-specific antigens (HNA), determine neutrophil destruction by complement-mediated lysis or splenic phagocytosis of antibody-coated neutrophils (3,4).
In 1975, the studies of Lalezari et al. and Boxer et al. firstly demonstrated the immunological basis of chronic neutropenia, showing the association of neutropenia with the presence of neutrophil-specific antibodies in the patients’ sera (5,6).
Neutrophil auto or alloantibodies can be responsible for a variety of clinical conditions in infancy including autoimmune neutropenia (AIN), which comprises post-infection neutropenia (PIN) and drug-induced neutropenia (DIN), alloimmune neonatal neutropenia (ANN), and neonatal alloimmune neutropenia secondary to maternal AIN. Although very infrequent in pediatric patients, febrile and severe pulmonary transfusion reactions, such as transfusion-related acute lung injury (TRALI) can be also caused by the presence of neutrophil alloantibodies (7).
After a brief description of these clinical conditions, this review will provide an overview of the laboratory diagnostic approach for the detection of neutrophil antibodies in immune pediatric neutropenias. We present the following article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-43/rc).
Methods
For the drafting of this review, a literature search of the last 10 years was carried out in PubMed. In addition, pivotal papers published before 2010 were considered (Table 1). The keywords used for the research were neonatal neutropenia, autoimmune neutropenia, granulocyte/neutrophil antibodies, granulocyte/neutrophil antigens, HNA system, and granulocyte serology.
Table 1
| Items | Specification |
|---|---|
| Date of search | 15/05/2020 |
| Databases and other sources searched | PubMed |
| Search terms used | Neonatal neutropenia, autoimmune neutropenia, granulocyte/neutrophil antibodies, granulocyte/neutrophil antigens, HNA system, granulocyte serology |
| Timeframe | 2010–2020; in addition, pivotal papers published before 2010 were considered |
| Inclusion and exclusion criteria | Referred paper (journal or conference), book chapter; English language only |
| Selection process | Selection was conducted by hand search, citation analysis and previously known articles plus approached authors |
Discussion
AIN
AIN can have a heterogeneous background, as an isolated disorder (primary AIN, pAIN), or occurring within the frame of other autoimmune diseases and even as a complication of infections, drugs, malignancy or vaccination (secondary AIN).
Primary AIN, also named chronic benign neutropenia of infancy and childhood, is one of the most relevant causes of neutropenia in children, usually occurring in infants before the age of 3 years and is most often diagnosed between 5 and 15 months of age (8).
This disease is associated with the development of neutrophil-specific autoantibodies. The mechanisms triggering the generation of these antibodies remain elusive. However, molecular mimicry of microbial antigens, drug modification of endogenous antigens, abnormal expression of HLA antigens, or failure to suppress self-reactive clones could be involved in this process (9). AIN overlaps with ANN and iso-immune neutropenia because the involved neutrophil-specific antigens are often the same (i.e., HNA1a, HNA-1b, and HNA 1c), but in AIN there are no neutrophil-specific antibodies in the maternal serum (10).
Due to the high false-negative rate of antibody screening, in some patients the AIN diagnosis can be missed. Such patients are generally indicated as suffering from “chronic idiopathic neutropenia” (CIN). Recently, a study of the Italian Neutropenia Registry on 157 pediatric patients with suspected AIN, reported a test sensitivity of 62% for neutrophil antibodies detection on the first screening; repeated evaluations showed 82% of positive results despite the clinical course of all patients was typical of primary AIN. In fact, about 90% of patients recovered within 2 years from diagnosis and serious infections were only present in 12–20% of affected children (8,11).
AIN associated with other autoimmune diseases is usually defined as secondary AIN. A significant paper by Marie Bruin and colleagues (12) showed that the secondary forms appeared at higher age and were characterized by a more severe clinical course with a lower tendency to spontaneous recovery, as compared to primary forms. Moreover, in patients with primary AIN, the neutrophil autoantibodies were frequently directed against one of the alloforms of the HNA-1 system, whereas in patients with secondary AIN, antibodies with pan-FcγRIIIb specificity were usually detected (8,12,13) (Table 2).
Table 2
| Clinical condition | Antibody specificity | Assays |
|---|---|---|
| ANN | HNA-1a, -1b, -1c†, -1d†, FcγRIIIb†, HNA-2, HNA-3a†, -3b†, HNA-4a†, -4b†, HNA-5a† | GIFT, fluorescent microbead test (Luminex) or MAIGA, PCR-SSP for HNA antigen typing |
| AIN | HNA-1a, -1b, -1c†, FcγRIIIb | GIFT, fluorescent microbead test (Luminex) or MAIGA |
| PIN | FcγRIIIb (pan reactive) | GIFT |
| DIN | FcγRIIIb, HNA-2 | GIFT (in the presence of drug) |
| TRALI | HNA-2, HNA-3a | GAT, GIFT, fluorescent microbead test (Luminex) or MAIGA |
Adapted from Flesch et al. (14). †, rare association or with limited cases reported in the literature. ANN, alloimmune neonatal neutropenia; AIN, autoimmune neutropenia; PIN, post-infection neutropenia; DIN, drug-induced neutropenia; HNA, human neutrophil antigens; TRALI, transfusion-related acute lung injury; GIFT, granulocyte immunofluorescence test; PCR, polymerase chain reaction; SSP, sequence-specific amplification; MAIGA, monoclonal antibody immobilization of granulocyte antigens; GAT, granulocyte agglutination test.
PIN is often antibody-mediated, and is very frequent in newborns and infants (15). It is generally related to bacterial or viral infections with no other evident causes. Generally, the duration is limited (less than 1–3 months) and the ANC normalizes with the resolution of the infection (15,16).
Although most cases of drug-induced granulocytopenia are due to direct bone marrow toxicity, immune-mediated processes may occasionally occur (17). Indeed, it has been shown that drug-dependent antibodies can bind to both mature granulocytes and their precursors (18). The main granulocyte glycoproteins involved in the mechanism leading to DIN are FcγRIIIb and CD177, which bind drugs and their metabolites and are therefore recognized as new antigens by drug-dependent neutrophil antibodies. The majority of patients recover after drug discontinuation (17,19-21).
To confirm the diagnosis, the patient’s sera should be tested in the absence and the presence of the drug and/or its metabolites, but this method lacks standardization. It should be noted that only a minority of DIN cases have been reported amongst children and young adults. Anti-epileptics are the medications most frequently implicated in DIN during infancy (22).
ANN
In ANN the mother becomes immunized to paternal inherited HNA present on fetal neutrophils. Maternal neutrophil-specific antibodies of IgG class transplacentally pass to the fetus (23,24) and bind to mature fetal neutrophils which, thereafter, are rapidly eliminated from the circulation by the spleen, liver and lungs. Neonates affected by ANN exhibit neutropenia at birth or within 1 to 3 days (23). ANN can occur in the first pregnancy and can have different clinical presentations, ranging from no symptoms to severe infections (23,25).
Although the clinical effects usually dissipate during the first 6 weeks of life, maternal antibodies can persist in the fetal circulation for up to 6 months (23).
Antibodies against HNA-1 isoforms are responsible for the majority of ANN cases. Rarely, mothers who are FcγRIIIb deficient (CD16 null phenotype) may produce anti-FcγRIIIb isoantibodies, which can result in isoimmune neonatal neutropenia (26).
Few cases of ANN associated with other HNA are reported in the literature (14,27-32) (Table 2).
Recently, in a large Brazilian cohort of patients with ANN, Abbas et al. showed a frequency of ANN in 8/10,000 neonates. Among the HNA antibodies identified, all cases were related to HNA-1/-3 systems. In cases with maternal-fetal incompatibility for HNA-4 and -5, no specific neutrophil alloantibodies were found, but anti-HLA of class I or II were present (33). These antibodies are commonly found in multiparous women and their newborns’ sera; however, HLA antibodies are not associated with neutropenia (1).
Moreover, few cases of neonatal neutropenia due to passive transfer of maternal circulating neutrophil-specific autoantibodies from mother with AIN have been described. Such neonates are neutropenic at birth, and the neutropenia can persist for weeks or months after delivery (34,35).
HNA system
HNA definition and associated nomenclature are regulated by the International Society of Blood Transfusion Granulocyte Immunology Working Party (ISBT GIWP) (36) and are based on serologically defined epitopes on the associated glycoproteins (37). Overall, five HNA systems (HNA-1, -2, -3, -4, and -5) have been described (38) (Table 3).
Table 3
| Antigens groups | Antigen/Epitope | Glycoprotein | Allele |
|---|---|---|---|
| HNA-1 | HNA-1a | CD16b | FCGR3B*01 FCGR3B*04 |
| HNA-1b, HNA-1c | CD16b | FCGR3B*02 | |
| HNA-1b, HNA-1d | CD16b | FCGR3B*03 | |
| HNA-1b variant | CD16b | FCGR3B*05 | |
| HNA-1 null | No glycoprotein | FCGR3B*null | |
| HNA-2 | HNA-2a | CD177 | |
| HNA-2 null | No glycoprotein | ||
| HNA-3 | HNA3a | CTL2 | SLC44A2*01 |
| HNA3b | CTL2 | SLC44A2*02 | |
| HNA-3a variant | SLC44A2*03 | ||
| HNA-4 | HNA-4a | CD11b | ITGAM*01 |
| HNA-4b | CD11b | ITGAM*02 | |
| HNA-5 | HNA-5a | CD11a | ITGAL*01 |
| ITGAL*02 |
HNA-1 isoforms (HNA-1a, -1b, -1c and -1d) are expressed only on neutrophils. These alleles differ from each other by only five single nucleotide polymorphisms (SNP) which result in four different amino acids. As shown in Table 3, there is no unique relationship between allele and antigen (40,41). Furthermore, individuals lacking FcγRIIIb with a CD16 null phenotype, although rare (42,43), have been identified. Therefore, the HNA-1 epitopes are not present on the neutrophils of these subjects (44).
The HNA-2 antigen (i.e., HNA-2a), located on a 58- to 64-kDa glycoprotein, can be present on both neutrophils and its precursors. HNA-2 has no antigenic diversity, although polymorphisms have been described (38). Different SNP have been attributed to the fact that HNA-2 expression can vary between individuals and that a percentage of positive and negative HNA-2 neutrophils can be found in the same subject (45,46). Moreover, as for HNA-1, HNA-2 null individuals have been reported, although their frequency is very low (38).
HNA-3 antigens (HNA-3a and -3b) are expressed on granulocytes, lymphocytes, platelets, endothelial cells, kidneys, spleen, and placental cells. They are located on choline transporter-like protein 2 (CTL2) (47,48) and appear to be encoded by an R>Q 154 amino acid substitution (49). Antibodies against HNA-3a, although rare, are occasionally implicated in immune neutropenia and serious and fatal events of TRALI (50-52).
HNA-4 (HNA-4a and -4b) and HNA-5 antigens are encoded by CD11b and CD11a integrins of the beta2 family (53). These molecules are adhesive receptors that are essential for cell-cell interactions and cell trafficking and their main role is immune activation and suppression (54).
HNA-4a is expressed on granulocytes, monocytes, and natural killer cells, whereas HNA-5a is expressed on all leukocytes (53).
Over the past decade several easy PCR assays have been implemented to define the HNA typing by the evaluation of HNA-1, -3, -4, and -5 alleles (55,56). Although the gene encoding for HNA-2 has already been described, standardized genotyping methods are not yet available (38). This is due to several mutations leading to different CD177 mRNA splicing defects (57).
Granulocyte antibody screening
Detection of anti-neutrophil antibodies is a useful diagnostic tool to define immune-mediated neutropenia.
Concerning the techniques used and when the antibody titer is low, the detection of these antibodies is often laborious, difficult, and with low sensitivity (8,58-60).
In 2013, the report of the ISBT on the quality assessment for the detection and identification of granulocyte antibodies underlined that the number of laboratories providing a diagnostic service for granulocyte antibody detection was few because of the technical difficulties, which include the scarcity of typing antisera, the access to HNA-typed donors and the requirement for freshly isolated granulocytes (59). Moreover, during the study period from 2000 to 2012, the overall rate of correct antibody identification was 83% for sera with HNA antibodies alone, and 80% for sera containing both HNA and HLA antibodies (59).
Since no single technique has been shown to consistently detect all clinically relevant granulocyte antibodies, the sensitivity of a particular test is difficult to assess. The heterogeneity and multifactorial etiology of suspected immune neutropenias hamper the evaluation of a diagnostic test specificity (61).
Generally, as with the other blood cells, i.e., platelets and erythrocytes, neutrophil antibodies can be searched directly bound to the patient’s cells (Direct test) and/or circulating in their own plasma (Indirect test). Still today there is much discussion about whether to perform the direct test for the evaluation of neutrophil antibodies in neutropenic patients.
Granulocyte immunofluorescence test (GIFT)
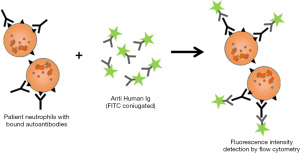
Developed by Verheugt et al. in the late 1970s, this fluorescent “antiglobulin” test is used for the detection of both circulating (Indirect GIFT) and granulocyte bound (Direct GIFT) allo and autoantibodies (62). This test can be evaluated by fluorescence microscopy or by flow cytometry.
Direct test (D-GIFT)
The D-GIFT reveals the amount of antibodies bound to the membrane of the patient’s neutrophils (12,63) (Figure 1).
In the literature, there are conflicting opinions regarding the usefulness of this test, mainly because the accuracy of this evaluation is often hampered by an insufficient number of target cells in severely neutropenic patients. Moreover, possible unspecific binding of immunocomplexes to the neutrophil Fcγ receptors can occur (8). The latter issue can be due to neutrophils’ activation, a condition that could occur during infections or G-CSF treatment (64). Therefore, D-GIFT has to be performed in patients without ongoing infections or under treatment with G-CSF, a drug frequently used in severe neutropenia cases (65). When these conditions happen, a positive D-GIFT is not indicative of a real presence of granulocyte-specific antibodies (62,66). On the contrary, a negative D-GIFT may presumably exclude AIN, since false negatives are rare (15).
As suggested by our work (58), D-GIFT, performed with adequate reference values and well-trained personnel, can improve the differential diagnosis of autoimmune versus non-autoimmune pediatric neutropenia. In fact, the performance of the D-GIFT allows the detection of neutrophil-bound antibodies in about 50% of patients with chronic benign neutropenia resulting negative in the indirect test (58). Therefore, using both tests, clinicians could confirm, in the first laboratory workup, the autoimmune etiology of neutropenia with 90% specificity. This would help avoid other invasive and expensive diagnostic procedures on the infant that may be necessary for suspected congenital neutropenia and neutropenia caused by bone marrow failure (60).
Indirect GIFT (I-GIFT)
The I-GIFT, developed in the late 1970s, is still performed today in the same way (Figure 2). Flow cytometry is now mainly used for fluorescence detection, instead of microscopy, thus allowing the objective analysis of much more cells, in a shorter time, with more sensitivity and reproducibility (23). For result evaluation, some laboratories take into account the difference, other than the ratio, between the median fluorescence intensity of a patient's serum and that of a negative serum on the same donor's neutrophils (58).

Even today, I-GIFT is considered the most sensitive assay for the detection of neutrophil antibodies since almost all HNA antibodies can be detected. It is also generally considered more sensitive than the granulocyte agglutination test (GAT) except for HNA-3 antibodies that showed only a weak reactivity (67).
The I-GIFT shows a very low false-positive rate, however, there is a significant frequency of false negatives (8). The low levels of circulating granulocyte-specific antibodies and the possible missing of uncommon HNA in the neutrophils’ panel used are the major causes of the limited sensitivity of the I-GIFT (9). As HNA-1a is the principal antigen involved in pediatric AIN, to improve antibody detection, the neutrophil panel should include at least one homozygous HNA-1a/1a and one HNA-1b/1b test cell, since the difficulty of autoantibody binding to granulocytes coming from HNA-1a/1b heterozygous donors is well known (60). Moreover, it is necessary to repeat the test several times before the antibody is successfully detected in a patient’s serum. In addition, this limit could be amplified by the different HNA-1 density on the neutrophils’ surface (68).
Overall, Bux and colleagues in their pivotal work (8), in which presented data of the largest cohort of AIN pediatric patients, estimated that by using both GAT and GIFT, in 74% of patients’ neutrophil antibodies can be identified at the first screening (1,4). More recently, we and others (58) detected neutrophil antibodies in 52% of suspected AIN patients at the first screening with GIFT and GAT, and reached a sensitivity of 84% only with repeated antibody testing.
GAT
The GAT was developed by Lalezari in the early 1960s (69). This test shows one of the functional outcomes of neutrophils in response to specific binding between antibodies and their antigenic epitopes. Granulocytes are firstly sensitized and subsequently move towards other granulocytes by the formation of pseudopods to form microscopic agglutinates, until membrane contact is established (70) (Figure 3). The reactions, evaluated by optical microscopy, are graded from negative to 4+ serological score based on the percentage of cells that are agglutinated. It’s important to keep in mind that agglutination is a dynamic process that requires intact cells. Low temperatures and disruption of cell metabolism or cytoskeleton functions will prevent this active agglutination (39). In experienced hands, the combination of GIFT and GAT, on a well-phenotyped cell panel, accurately identifies the specificity and clinical significance of granulocytes reactive antibodies (59).

In our series, detection of agglutinating antibodies by GAT did not improve the diagnostic accuracy (58).
Monoclonal antibody immobilization of granulocyte antigens (MAIGA)
The MAIGA assay, similarly to the detection and characterization of platelet alloantibodies (MAIPA) (71), allows the identification of serum antibody specificities based on the immobilization of neutrophil antigens captured in a wells plate by monoclonal antibodies specific for the carrier glycoprotein (Figure 4) (72,73). MAIGA is still considered the gold standard assay for the identification of granulocyte antibodies, having the peculiarity to detect HNA antibodies even in the presence of HLA antibodies without the need to pre-absorb sera with platelet pools (74). However, this test has some critical issues: first of all, it is a quite complex procedure that requires highly trained personnel (15); furthermore, the choice of clones of monoclonal antibodies is critical to avoid false-negative results due to steric hindrance and competition for the antibody binding site. Finally, a large amount of typed granulocytes is needed (and therefore large volumes of blood taken from typed donors) and it is often difficult to find donors with granulocytes expressing rare HNA antigens (75).
Fluorescence bead test
Recently, the microbead assay LABScreen (One Lambda Inc. Canoga Park, CA, USA), a solid phase assay based on fluorescent-labeled beads, was introduced to overcome the traditional limitations of the classical serological methods.
Initially applicable only to HLA class I and II antibodies, this assay was subsequently implemented for the detection of HNA antibodies (LABScreen MULTI) and during the last years, this test is often used in association with GIFT particularly to identify antibody specificity (76,77).
The principle of this assay is based on the use of a mixture of beads coated with HNA or HLA class I or II peptides. The antibodies present in sera or plasma samples bind the specific peptide antigen present on the beads and are detected by labeling with a secondary antibody conjugated with phycoerythrin (PE)-labeled anti-human globulin which is visualized in the Luminex flow system.
Initial studies conducted on sera from ANN, AIN, and TRALI cases using the first generation LABScreen MULTI, evidenced a good concordance with I-GIFT (94%) in HNA-1 and HNA-2 alloantibody detection. On the other hand, autoantibody detection in AIN was not so concordant with classical methods both in terms of sensitivity and specificity. Therefore, nowadays this assay should be used cautiously in suspected AIN (76).
With the new-generation LABScreen MULTI assay, which includes the detection of HNA-3, -4, and -5 epitopes, the false positive rate is estimated to be around 5.5%. However, the problem of sensitivity remains, particularly for HNA-3 antibodies which usually react with antigens correctly expose on the cell membrane in their unmodified conformation. Probably, recombinant HNA-3 epitopes need to be modified to better mimic the natural protein conformation (77).
Despite actual limitations, LABScreen MULTI is currently the only commercial method available for HNA antibody screening. This platform can be fully automated, therefore allowing for large-scale testing of patients or blood donors. Hopefully, in the future this assay will improve sensitivity and specificity and offer a cost-effective diagnostic screening platform for HNA antibodies (78).
Our recent experience with this assay shows that anti-neutrophil antibodies with broad specificity (i.e., FcγRIIIb), often present in secondary AIN or in PIN and detected by I-GIFT, may not be found with this assay. In addition, we unexpectedly detected anti HNA-4a antibodies in sera of non-neutropenic patients (data unpublished).
Nowadays, GIFT and GAT performed on whole cells bearing native and unmodified antigenic structures, remain the gold standard methods for the screening of rare and currently unknown HNA specificities. Moreover, the specific HNA target of several autoantibodies has not been yet defined, thus the use of specific recombinant protein results, in these cases, unsuitable for diagnostic workup (38).
HNA transfected cell lines
Another strategy to avoid the use of freshly separated granulocytes for antibody screening is the development of cell lines expressing HNA antigens. The first attempt was made by Bux et al. in 1999, who developed cell lines stably expressing the HNA-1a, -1b, and -1c through the transfection of specific cDNA into Chinese hamster ovary cells. These cell lines had good stability (1 month at 4 ℃) but revealed a high fluorescence background when evaluated with GIFT assay (79).
More recently, Yasui et al. transduced selected HNA cDNA with a retroviral vector to confer a stable expression of HNA in erythroleukemia K562 cell lines, expressing a low level of natural leukocyte antigens and a very low background reactivity when tested with control sera. These cell lines are stable for one year while being maintained in culture (80,81). Serological evaluations, performed in a reference laboratory, revealed a good concordance with the standard GIFT assay (82).
Stable HNA-expressing cell lines potentially represent a useful alternative to fresh cells for the standard GIFT in reference laboratories. However, more experiments using these cell lines are needed to determine real advantages in granulocyte serology for diagnostic applications.
Laboratory management of immune neutropenias
According to the recommendations of the ISBT GIWP (83), the work up of suspected immune neutropenia cases should consist of a combination of at least two tests, one for the detection (GIFT and GAT) and another (MAIGA or a bead-based assay) for the identification of serum granulocyte antibodies.
Several laboratories also perform a direct test for the evaluation of neutrophil-bound immunoglobulins. These evaluations have to be repeated at least four times a year before excluding the presence of anti-neutrophil antibodies (15,84).
If neutropenia occurs in the first days of an infant’s life, neonatal alloimmune neutropenia may be suspected (ANN). Laboratory workup of suspected ANN includes the same tests of AIN performed on both patient’s and mother’s samples. Furthermore, a cross-match between patient’s or father’s neutrophils and maternal serum, usually performed by GIFT, could be of interest when HNA epitopes are not included in the neutrophil panel.
Finally, HNA genotyping of both parents and neonate is carried out to verify the presence of a mismatch between the patient’s antigenic pattern and the maternal one (85). A schematic algorithm of laboratory investigations to be performed in cases of suspected ANN is depicted in Figure 5.

Conclusions
Antibody testing and HNA typing techniques are useful in the diagnosis of immune neonatal and infant neutropenias. However, the access to these diagnostic tools is not straightforward due to the low number of specialized laboratories with adequate skills, experience, and knowledge of the clinical and molecular aspects of granulocyte immunology. New commercial assays which do not require fresh cells are expected to improve test standardization, and could also be introduced in non-specialized laboratories. However, they still need to complete the necessary validations to replace reliable, home-made serological methods.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-43/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-43/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dinauer M. The phagocyte system and disorders of granulopoiesis and granulocyte function. In: Nathan D, Orkin S. editors. Nathan and Oshi’s Hematology of Infancy and Childhood. Philadelphia, PA: WBS Saunders, 2003:923-1010.
- Hsieh M, Chin K, Link B, et al. Benign Ethnic Neutropenia in Individuals of African Descent: Incidence, Granulocyte Mobilization, and Gene Expression Profiling. Blood 2005;106:3069. [Crossref]
- Dale DC. How I manage children with neutropenia. Br J Haematol 2017;178:351-63. [Crossref] [PubMed]
- Papadaki HA, Pontikoglou C. Pathophysiologic mechanisms, clinical features and treatment of idiopathic neutropenia. Expert Rev Hematol 2008;1:217-29. [Crossref] [PubMed]
- Lalezari P, Jiang AF, Yegen L, et al. Chronic autoimmune neutropenia due to anti-NA2 antibody. N Engl J Med 1975;293:744-7. [Crossref] [PubMed]
- Boxer LA, Greenberg MS, Boxer GJ, et al. Autoimmune neutropenia. N Engl J Med 1975;293:748-53. [Crossref] [PubMed]
- Reil A, Keller-Stanislawski B, Günay S, et al. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox Sang 2008;95:313-7. [Crossref] [PubMed]
- Bux J, Behrens G, Jaeger G, et al. Diagnosis and clinical course of autoimmune neutropenia in infancy: analysis of 240 cases. Blood 1998;91:181-6. [Crossref] [PubMed]
- Capsoni F, Sarzi-Puttini P, Zanella A. Primary and secondary autoimmune neutropenia. Arthritis Res Ther 2005;7:208-14. [Crossref] [PubMed]
- Dale DC, Bolyard AA. An update on the diagnosis and treatment of chronic idiopathic neutropenia. Curr Opin Hematol 2017;24:46-53. [Crossref] [PubMed]
- Fioredda F, Calvillo M, Burlando O, et al. Infectious complications in children with severe congenital, autoimmune or idiopathic neutropenia: a retrospective study from the Italian Neutropenia Registry. Pediatr Infect Dis J 2013;32:410-2. [Crossref] [PubMed]
- Bruin MC, von dem Borne AE, Tamminga RY, et al. Neutrophil antibody specificity in different types of childhood autoimmune neutropenia. Blood 1999;94:1797-802. [Crossref] [PubMed]
- Bussel JB, Abboud MR. Autoimmune neutropenia of childhood. Crit Rev Oncol Hematol 1987;7:37-51. [Crossref] [PubMed]
- Fung YL, Pitcher LA, Willett JE, et al. Alloimmune neonatal neutropenia linked to anti-HNA-4a. Transfus Med 2003;13:49-52. [Crossref] [PubMed]
- Farruggia P, Dufour C. Diagnosis and management of primary autoimmune neutropenia in children: insights for clinicians. Ther Adv Hematol 2015;6:15-24. [Crossref] [PubMed]
- Alexandropoulou O, Kossiva L, Haliotis F, et al. Transient neutropenia in children with febrile illness and associated infectious agents: 2 years' follow-up. Eur J Pediatr 2013;172:811-9. [Crossref] [PubMed]
- Stroncek DF. Drug-induced immune neutropenia. Transfus Med Rev 1993;7:268-74. [Crossref] [PubMed]
- Curtis BR. Non-chemotherapy drug-induced neutropenia: key points to manage the challenges. Hematology Am Soc Hematol Educ Program 2017;2017:187-93. [Crossref] [PubMed]
- Nagalapuram V, McCall D, Palabindela P, et al. Outcomes of Isolated Neutropenia Referred to Pediatric Hematology-Oncology Clinic. Pediatrics 2020;146:e20193637. [Crossref] [PubMed]
- Curtis BR. Drug-induced immune neutropenia/agranulocytosis. Immunohematology 2014;30:95-101. [Crossref] [PubMed]
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 2004;100:228-37. Erratum in: Cancer 2004;100:1993-4. [Crossref] [PubMed]
- Andrès E, Maloisel F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol 2008;15:15-21. [Crossref] [PubMed]
- Clay ME, Schuller RM, Bachowski GJ. Granulocyte serology: current concepts and clinical signifcance. Immunohematology 2010;26:11-21. [Crossref] [PubMed]
- Lalezari P, Nussbaum M, Gelman S, et al. Neonatal neutropenia due to maternal isoimmunization. Blood 1960;15:236-43. [Crossref] [PubMed]
- Lalezari P. Alloimmune neonatal neutropenia. In: Engelfriet CP, Van Loghum JJ, von dem Borne AEG. editors. Immunohaematology. Amsterdam: Elsevier Science Publishers, 1984;179-86.
- Maślanka K, Guz K, Uhrynowska M, et al. Isoimmune neonatal neutropenia due to anti-Fc(gamma) RIIIb antibody in a mother with an Fc(gamma) RIIIb deficiency. Transfus Med 2001;11:111-3. [Crossref] [PubMed]
- Lopes LB, Abbas SA, Moritz E, et al. Antibodies to human neutrophil antigen HNA-3b implicated in cases of neonatal alloimmune neutropenia. Transfusion 2018;58:1264-70. [Crossref] [PubMed]
- Curtis BR, Roman AS, Sullivan MJ, et al. Two cases of maternal alloimmunization against human neutrophil alloantigen-4b, one causing severe alloimmune neonatal neutropenia. Transfusion 2016;56:101-6. [Crossref] [PubMed]
- Mraz GA, Crighton GL, Christie DJ. Antibodies to human neutrophil antigen HNA-4b implicated in a case of neonatal alloimmune neutropenia. Transfusion 2016;56:1161-5. [Crossref] [PubMed]
- Porcelijn L, Abbink F, Terraneo L, et al. Neonatal alloimmune neutropenia due to immunoglobulin G antibodies against human neutrophil antigen-5a. Transfusion 2011;51:574-7. [Crossref] [PubMed]
- de Haas M, Muniz-Diaz E, Alonso LG, et al. Neutrophil antigen 5b is carried by a protein, migrating from 70 to 95 kDa, and may be involved in neonatal alloimmune neutropenia. Transfusion 2000;40:222-7. [Crossref] [PubMed]
- van den Tooren-de Groot R, Ottink M, Huiskes E, et al. Management and outcome of 35 cases with foetal/neonatal alloimmune neutropenia. Acta Paediatr 2014;103:e467-74. [Crossref] [PubMed]
- Abbas SA, Lopes LB, Moritz E, et al. Serologic and molecular studies to identify neonatal alloimmune neutropenia in a cohort of 10,000 neonates. Br J Haematol 2021;192:778-84. [Crossref] [PubMed]
- Fung YL, Pitcher LA, Taylor K, et al. Managing passively acquired autoimmune neonatal neutropenia: a case study. Transfus Med 2005;15:151-5. [Crossref] [PubMed]
- Seguier J, Barlogis V, Croisille L, et al. Severe Transitory Neonatal Neutropenia Associated with Maternal Autoimmune or Idiopathic Neutropenia. J Clin Immunol 2019;39:200-6. [Crossref] [PubMed]
- Flesch BK, Curtis BR, de Haas M, et al. Update on the nomenclature of human neutrophil antigens and alleles. Transfusion 2016;56:1477-9. [Crossref] [PubMed]
- Flesch B. Work-up in the case of granulocyte antibodies. ISBT Sci Ser 2020;15:59-69. [Crossref]
- Browne T, Dearman RJ, Poles A. Human neutrophil antigens: Nature, clinical significance and detection. Int J Immunogenet 2021;48:145-56. [Crossref] [PubMed]
- von dem Borne AE, de Haas M, Roos D, et al. Neutrophil antigens, from bench to bedside. Immunol Invest 1995;24:245-72. [Crossref] [PubMed]
- Bux J. Molecular nature of granulocyte antigens. Transfus Clin Biol 2001;8:242-7. [Crossref] [PubMed]
- Reil A, Sachs UJ, Siahanidou T, et al. HNA-1d: a new human neutrophil antigen located on Fcγ receptor IIIb associated with neonatal immune neutropenia. Transfusion 2013;53:2145-51. [Crossref] [PubMed]
- Cardoso SP, Chong W, Lucas G, et al. Determination of human neutrophil antigen-1, -3, -4 and -5 allele frequencies in English Caucasoid blood donors using a multiplex fluorescent DNA-based assay. Vox Sang 2013;105:65-72. [Crossref] [PubMed]
- Porretti L, Cattaneo A, Coluccio E, et al. Implementation and outcomes of a transfusion-related acute lung injury surveillance programme and study of HLA/HNA alloimmunisation in blood donors. Blood Transfus 2012;10:351-9. [PubMed]
- Stroncek DF, Skubitz KM, Plachta LB, et al. Alloimmune neonatal neutropenia due to an antibody to the neutrophil Fc-gamma receptor III with maternal deficiency of CD16 antigen. Blood 1991;77:1572-80. [Crossref] [PubMed]
- Li Y, Mair DC, Schuller RM, et al. Genetic mechanism of human neutrophil antigen 2 deficiency and expression variations. PLoS Genet 2015;11:e1005255. [Crossref] [PubMed]
- Flesch BK, Reil A. Molecular Genetics of the Human Neutrophil Antigens. Transfus Med Hemother 2018;45:300-9. [Crossref] [PubMed]
- Van Leeuwen A, Eernisse JG, Van Rood JJ. A New Leucocyte Group with Two Alleles: Leucocyte Group Five. Vox Sang 1964;9:431-46. [Crossref] [PubMed]
- Greinacher A, Wesche J, Hammer E, et al. Characterization of the human neutrophil alloantigen-3a. Nat Med 2010;16:45-8. [Crossref] [PubMed]
- Curtis BR, Cox NJ, Sullivan MJ, et al. The neutrophil alloantigen HNA-3a (5b) is located on choline transporter-like protein 2 and appears to be encoded by an R>Q154 amino acid substitution. Blood 2010;115:2073-6. [Crossref] [PubMed]
- Kopko PM, Marshall CS, MacKenzie MR, et al. Transfusion-related acute lung injury: report of a clinical look-back investigation. JAMA 2002;287:1968-71. [Crossref] [PubMed]
- Davoren A, Curtis BR, Shulman IA, et al. TRALI due to granulocyte-agglutinating human neutrophil antigen-3a (5b) alloantibodies in donor plasma: a report of 2 fatalities. Transfusion 2003;43:641-5. [Crossref] [PubMed]
- Reil A, Wesche J, Greinacher A, et al. Geno- and phenotyping and immunogenicity of HNA-3. Transfusion 2011;51:18-24. [Crossref] [PubMed]
- Arnaout MA. Biology and structure of leukocyte β 2 integrins and their role in inflammation. F1000Res. 2016;5:F1000 Faculty Rev-2433.
- Fagerholm SC, Guenther C, Llort Asens M, et al. Beta2-Integrins and Interacting Proteins in Leukocyte Trafficking, Immune Suppression, and Immunodeficiency Disease. Front Immunol 2019;10:254. [Crossref] [PubMed]
- Veldhuisen B, Porcelijn L, Ellen van der Schoot C, et al. Molecular typing of human platelet and neutrophil antigens (HPA and HNA). Transfus Apher Sci 2014;50:189-99. [Crossref] [PubMed]
- Steffensen R, Baech J, Nielsen KR. Allelic Discrimination by TaqMan-PCR for Genotyping of Human Neutrophil Antigens. Methods Mol Biol 2015;1310:205-12. [Crossref] [PubMed]
- Kissel K, Scheffler S, Kerowgan M, et al. Molecular basis of NB1 (HNA-2a, CD177) deficiency. Blood 2002;99:4231-3. [Crossref] [PubMed]
- Porretti L, Farruggia P, Colombo FS, et al. Diagnostic value of cell bound and circulating neutrophil antibody detection in pediatric neutropenia. Pediatr Blood Cancer 2018; [Crossref] [PubMed]
- Lucas G, Porcelijn L, Fung YL, et al. External quality assessment of human neutrophil antigen (HNA)-specific antibody detection and HNA genotyping from 2000 to 2012. Vox Sang 2013;105:259-69. [Crossref] [PubMed]
- Bruin M, Dassen A, Pajkrt D, et al. Primary autoimmune neutropenia in children: a study of neutrophil antibodies and clinical course. Vox Sang 2005;88:52-9. [Crossref] [PubMed]
- Shastri KA, Logue GL. Autoimmune neutropenia. Blood 1993;81:1984-95. [Crossref] [PubMed]
- Verheugt FW, von dem Borne AE, van Noord-Bokhorst JC, et al. Autoimmune granulocytopenia: the detection of granulocyte autoantibodies with the immunofluorescence test. Br J Haematol 1978;39:339-50. [Crossref] [PubMed]
- Ito T, Taniuchi S, Tsuji S, et al. Diagnosis of autoimmune neutropenia by neutrophil-bound IgG and IgM antibodies. J Pediatr Hematol Oncol 2011;33:552-5. [Crossref] [PubMed]
- Kuijpers TW, de Haas M, de Groot CJ, et al. The use of rhG-CSF in chronic autoimmune neutropenia: reversal of autoimmune phenomena, a case history. Br J Haematol 1996;94:464-9. [Crossref] [PubMed]
- Donini M, Fontana S, Savoldi G, et al. G-CSF treatment of severe congenital neutropenia reverses neutropenia but does not correct the underlying functional deficiency of the neutrophil in defending against microorganisms. Blood 2007;109:4716-23. [Crossref] [PubMed]
- Badolato R, Fontana S, Notarangelo LD, et al. Congenital neutropenia: advances in diagnosis and treatment. Curr Opin Allergy Clin Immunol 2004;4:513-21. [Crossref] [PubMed]
- Bux J. Human neutrophil alloantigens. Vox Sang 2008;94:277-85. [Crossref] [PubMed]
- Willcocks LC, Lyons PA, Clatworthy MR, et al. Copy number of FCGR3B, which is associated with systemic lupus erythematosus, correlates with protein expression and immune complex uptake. J Exp Med 2008;205:1573-82. [Crossref] [PubMed]
- Lalezari P, Pryce SC, Rose NR, et al. Detection of Neutrophil and Platelet Antibodies in Immunologically Induced Neutropenia and Thrombocytopenia; Manual of Clinical Immunology. Washington: American Society of Microbiology, 1980:744-9.
- Verheugt FW, von dem Borne AE, Décary F, et al. The detection of granulocyte alloantibodies with an indirect immunofluorescence test. Br J Haematol 1977;36:533-44. [Crossref] [PubMed]
- Kiefel V, Santoso S, Weisheit M, et al. Monoclonal antibody--specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood 1987;70:1722-6. [Crossref] [PubMed]
- Bux J, Kober B, Kiefel V, et al. Analysis of granulocyte-reactive antibodies using an immunoassay based upon monoclonal-antibody-specific immobilization of granulocyte antigens. Transfus Med 1993;3:157-62. [Crossref] [PubMed]
- Minchinton RM, Noonan K, Johnson TJ. Examining technical aspects of the monoclonal antibody immobilisation of granulocyte antigen assay. Vox Sang 1997;73:87-92. [Crossref] [PubMed]
- Fung YL, Goodison KA, Wong JK, et al. Investigating transfusion-related acute lung injury (TRALI). Intern Med J 2003;33:286-90. [Crossref] [PubMed]
- Simtong P, Romphruk AV, Hofmann C, et al. Improvement of monoclonal antibody-immobilized granulocyte antigen assay for the detection of anti-HNA-1 alloantibodies. Transfusion 2018;58:200-7. [Crossref] [PubMed]
- Fromont P, Prié N, Simon P, et al. Granulocyte antibody screening: evaluation of a bead-based assay in comparison with classical methods. Transfusion 2010;50:2643-8. [Crossref] [PubMed]
- Schulz U, Reil A, Kiefel V, et al. Evaluation of a new microbeads assay for granulocyte antibody detection. Transfusion 2017;57:70-81. [Crossref] [PubMed]
- Fung YL, Minchinton RM, Fraser JF. Neutrophil antibody diagnostics and screening: review of the classical versus the emerging. Vox Sang 2011;101:282-90. [Crossref] [PubMed]
- Bux J, Kissel K, Hofmann C, et al. The use of allele-specific recombinant Fc gamma receptor IIIb antigens for the detection of granulocyte antibodies. Blood 1999;93:357-62. [Crossref] [PubMed]
- Yasui K, Miyazaki T, Matsuyama N, et al. Establishment of cell lines stably expressing HNA-1a, -1b, and -2a antigen with low background reactivity in flow cytometric analysis. Transfusion 2007;47:478-85. [Crossref] [PubMed]
- Yasui K, Hirayama F, Matsuyama N, et al. New cell lines express HNA-1c, -4a, -4b, -5a, or -5b for identification of HNA antibodies. Transfusion 2008;48:1037-9. [Crossref] [PubMed]
- Lopez GH, Dean MM, Yasui K, et al. A standardized immunofluorescence test method with human neutrophil antigen-expressing cell lines to enhance antibody detection. Vox Sang 2012;102:171-4. [Crossref] [PubMed]
- ISBT Working Party on Granulocyte Immunobiology. Recommendations of the ISBT Working Party on Granulocyte Immunobiology for leucocyte antibody screening in the investigation and prevention of antibody-mediated transfusion-related acute lung injury. Vox Sang 2009;96:266-9. [Crossref] [PubMed]
- Fioredda F, Calvillo M, Bonanomi S, et al. Congenital and acquired neutropenia consensus guidelines on diagnosis from the Neutropenia Committee of the Marrow Failure Syndrome Group of the AIEOP (Associazione Italiana Emato-Oncologia Pediatrica). Pediatr Blood Cancer 2011;57:10-7. [Crossref] [PubMed]
- Porcelijn L, de Haas M. Neonatal Alloimmune Neutropenia. Transfus Med Hemother 2018;45:311-6. [Crossref] [PubMed]
Cite this article as: Cattaneo A, Porretti L. Laboratory testing for the diagnosis of neonatal and pediatric immune neutropenias: a narrative review. Pediatr Med 2022;5:30.