
A narrative review of Hyporeninemic hypertension—an indicator for monogenic forms of hypertension
Introduction
Hypertension is one of the leading causes of morbidity and mortality among adults with an estimated prevalence of 1.1 billion worldwide contributing to over 7 million deaths (1-6). Although the adverse outcomes of hypertension such as cardiovascular disease, stroke, and kidney disease usually manifest during adulthood, high blood pressure during childhood is progressive and one of the strongest predictors of adulthood hypertension in the future (7-9). High blood pressure in children is defined based on the child’s age, sex, and height according to the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents which was published in 2004 (10) and revised in 2017 (11). The prevalence of hypertension ranges from 2.2% to 11.4% in the general pediatric population (12,13) up to 24.8% in high-risk groups such as obese patients or kidney patients (14).
Primary hypertension is polygenic and results from complex interactions among different genes and environmental factors (15). On the other hand, secondary hypertension can be caused by a large number of different conditions that can be either acquired secondary to medications or related to renovascular, endocrine, oncologic, or neurological problems (16) (Table 1). There is a subcategory of secondary hypertension characterized by low renin and are referred to as hyporeninemic hypertension or low-renin hypertension (17) (Table 2). These forms of hypertension are caused by pathogenic variants in a single gene and are referred to as monogenic hypertension (18). Most of these genes are involved in the renal and adrenal regulation of blood pressure and intravascular volume. The net result of these mutations lead to either one or more of the following mechanisms: (I) increased mineralocorticoid synthesis, (II) increased response to normal/low mineralocorticoid levels, and (III) excessive sodium (Na+) reabsorption at the level of the nephrons. All three mechanisms result in volume excess and inability to excrete excess sodium (19). Recent advancement in genetic studies revealed that this form of hypertension is underdiagnosed in patients with hypertension (20,21). Unfortunately, the suppressed levels of renin and/or aldosterone are often overlooked and underappreciated as indicators for forms of monogenic hypertension. Moreover, the aldosterone-to-renin ratio provides an indicator of aldosterone excess even in patients with normal aldosterone levels (22). Importantly, once the specific cause is identified targeted treatment may allow for much better controlled blood pressures. The identification of hyporeninemic hypertension and then the correct diagnosis of the specific condition are crucial for the appropriate treatment. In this article, we outline the different conditions, their genetic causes, how to diagnose them, and how to treat the specific conditions.
Table 1
| Age | Primary or secondary | Causes for secondary hypertension |
|---|---|---|
| Birth to 1 year | Secondary (99%) | Neonatal: Maternal hypertension, maternal substance abuse, antenatal steroids, maternal obesity, and maternal diabetes mellitus, low birthweight, hypoxia, prematurity |
| Medications: steroids, indomethacin, vasopressors, bronchodilators, theophylline, caffeine, vitamin D intoxication, pancuronium, erythropoietin | ||
| Neurologic: seizures, increased intracranial pressure, intracranial hemorrhage, pain | ||
| Renal: renal parenchymal disease, congenital nephrotic syndrome, ARPKD, renovascular defect, cortical necrosis, pseudohypoaldosteronism type II Pulmonary: bronchopulmonary dysplasia |
||
| Endocrine: congenital adrenal hyperplasia, hyperthyroidism, hyperaldosteronism, adrenal hemorrhage | ||
| Neoplasia: Wilms tumor, Neuroblastoma, mesoblastic nephroma | ||
| Cardiac: coarctation of aorta, patent ductus arteriosus | ||
| Age 1–12 years | Secondary (70–85%); primary (15–30%) | Medications: steroids, NSAIDs, vasopressors, bronchodilators, vitamin D intoxication, pancuronium, erythropoietin, herbal medications, decongestants, oral contraception, cyclosporine, tacrolimus, ADHD medications |
| Neurologic: seizures, increased intracranial pressure, intracranial hemorrhage, pain | ||
| Renal: acute kidney injury, tuberous sclerosis, multicystic-dysplastic kidneys, obstructive uropathy, reflux uropathy, renal hypoplasia, interstitial nephritis, pyelonephritis, cortical necrosis, hypercalcemia | ||
| Renovascular disease: renal artery stenosis, renal venous thrombosis, midaortic syndrome, vascular compression | ||
| Endocrine: hyperthyroidism, hyperaldosteronism, congenital adrenal hyperplasia | ||
| Neoplasia: Wilms tumor, pheochromocytoma | ||
| Cardiac: coarctation of aorta | ||
| Age 12–18 years | Primary (85–95%); secondary (5–15%) | Same causes as in the 1–12 years group |
NSAIDs, non-steroidal anti-inflammatory drugs; ADHD, attention deficit hyperactivity disorder.
Table 2
| Monogenic HTN (low renin) | Aldosterone level | Genetic mutation | Mechanism | Inheritance | Electrolyte derangement | Management |
|---|---|---|---|---|---|---|
| Liddle syndrome | Low | SCNN1B, SCNN1G | Increased ENaC expression and activity Decreased ENaC degradation |
AD | Hypokalemia; metabolic alkalosis | Amiloride; triamterene; sodium restriction |
| Gordon syndrome (PHAII) | Normal to high | WNK-1, WNK-4, KLHL3, CUL3 | Increased NCC activity Increased ROMK channel internalization |
AD | Hyperkalemia, metabolic acidosis | Thiazide; sodium restriction; potassium restriction |
| Apparent mineralocorticoid excess (AME) | Low | HSD11B2 | Inactivation of 11HD2 Increased cortisone binding to MR |
AR | Hypokalemia; metabolic alkalosis | Spironolactone; eplerone; sodium restriction |
| Hypertension exacerbated by pregnancy (Geller syndrome) | Low | NR3C2 | Activation of MR | AD | Hypokalemia; metabolic alkalosis | Thiazide; amiloride; sodium restriction; avoid MR antagonists |
| Glucocorticoid-remediable aldosteronism (FH-1) | High | CYP11B1/CYP11B2 cross over | ACTH mediated- aldosterone production | AD | ± Hypokalemia; ± metabolic alkalosis | Low dose steroid; amiloride; spironolactone |
| FH-2 | High | CLCN2 | Depolarization of granulosa cells | AD | ± Hypokalemia; ± metabolic alkalosis | Adrenalectomy; amiloride; spironolactone |
| FH-3 | High | KCNJ5 | Depolarization of granulosa cells | AD | ± Hypokalemia; ± metabolic alkalosis | Adrenalectomy; amiloride; spironolactone |
| FH-4 | High | CACNA1H | Increased calcium influx in granulosa cells | AD | ± Hypokalemia; ± metabolic alkalosis | Adrenalectomy; amiloride; spironolactone |
ENaC, amiloride sensitive-epithelial (Na+) channel; NCC, sodium chloride cotransporter; PHAII, pseudohypoaldosteronism type II; ROMK, renal outer medullary potassium (K+) channel; WNK, with no lysine serine/threonine protein kinases gene; KLHL3, Kelch-like 3 gene; CUL3, Cullin 3 gene; 11HD2, 11β-hydroxysteroid dehydrogenase type 2 enzyme; FH, familial hyperaldosteronism; CYP11B1, 11-β- hydroxylase gene; CYP11B2, Aldosterone synthase gene; CLCN2, voltage gated chloride channel gene; KCNJ5, inward rectifying K+ channel gene.
Methods
We searched publications in PubMed online (www.pubmed.org) using title and abstract keywords “hyporeninemic hypertension”, “low renin hypertension”, “Liddle Syndrome”, “Gordon Syndrome”, “Apparent Mineralocorticoid Excess”, “Geller Syndrome”, “hypertension exacerbated by pregnancy”, “Glucocorticoid Remediable Aldosteronism”, “Familial Hyperaldosteronism”, “monogenic hypertension”, “inherited hypertension”, “genetic hypertension” (Table 3). We focused our search on the pediatric population. For literature on pathophysiology, we focused our search on the past 5 years. For the different syndromes, we referred to the original studies that provided early characterization of the disease.
Table 3
| Items | Specification |
|---|---|
| Date of search | 24th April, 2021 |
| Databases and other sources searched | PubMed |
| Search terms used | Hyporeninemic hypertension, monogenic hypertension, genetic hypertension, low renin hypertension, inherited hypertension, Liddle syndrome, Gordon syndrome, Apparent Mineralocorticoid Excess, Geller syndrome, primary hyperaldosteronism, familial hyperaldosteronism, Glucocorticoid Remediable Aldosteronism, congenital adrenal hyperplasia, familial glucocorticoid resistance |
| Timeframe | We did not apply a timeframe, but we focused on the last 5 years and pediatric cases; for pathophysiology we concentrated on the most recent publications |
| Inclusion and exclusion criteria | Inclusion criteria: publications in English language and focus on pediatric population |
| Selection process | Selection of references was agreed on by both authors |
| Any additional considerations, if applicable | Not applicable |
The renin-angiotensin-aldosterone system (RAAS)
The RAAS system has been extensively studied for its role in maintaining fluid homeostasis and blood pressure control (23). Upregulation of renin and aldosterone activity due to renal scarring, frequent UTIs, and chronic kidney disease is a major cause for hypertension with kidney disease (24). In addition to fluid balance and blood pressure control, RAAS plays an important role in chronic kidney disease progression (25), cardiovascular remodeling (26), activation of the thirst center and sympathetic nervous system (27), obesity and metabolic syndrome, inflammation and immune activation (18), lung (28), and liver fibrosis (29). Renin is an aspartyl-protease produced by the juxtaglomerular cells and the collecting duct. It converts angiotensinogen, a 14 amino acid peptide produced mainly in the liver, into angiotensin I (Ang-I), a 10 amino acid peptide (30). Renin secretion is regulated by mechanisms that reflect changes in blood volume and subsequently glomerular filtration (31). This includes the kidney baroreflex and chloride (Cl−) delivery at the level of the macula densa in addition to vasoactive molecules such as nitric oxide, adenosine, and prostaglandins (32). Ang-I has several ensuing fates that depend on the type of angiotensin converting enzymes present. This has been thoroughly reviewed by Silva et al. in the paper discussing the interaction of RAAS with coronavirus (33). In the classical pathway, Ang-I is cleaved by angiotensin converting enzyme (ACE), a dicarboxypeptidase located on the surface of endothelial cells mainly in the pulmonary and intestinal capillaries (34), into an 8 amino acid peptide angiotensin II (Ang-II). Ang-II is the effector molecule of the classical pathway. It has opposing effects depending on the receptor it binds to. Activation of angiotensin receptor type 1 (AT1R) will overall lead to increase in blood pressure, sodium retention, inflammation, remodeling, and fibrosis mediated by vasoconstriction, stimulation of aldosterone production and anti-diuretic hormone (ADH) production, and activation of sympathetic nervous system (SNS) tone (26). On the other hand, activation of the Angiotensin Receptor type 2 (AT2R) has an opposite effect that results in lowering blood pressure and increased sodium excretion. However, AT2R expression is significant during fetal development and early neonatal life (31). In the alternative pathway, Ang-I is cleaved by ACE2, a monocarboxypeptidase integral membrane protein (35). ACE2 is cleaved by ADAM17, a metalloprotease, and released into the blood stream. It cleaves Ang-I to generate Ang-(1-7), a heptapeptide, that preferentially binds to the Mas receptor (28). Although the downstream molecular pathways of Mas receptor activation are not fully understood, most of the experimental studies showed an overall counter Ang-II effect including vasodilation, decreased aldosterone and anti-diuretic hormone production, decreased inflammation and reactive oxygen species production (36,37). Due to the COVID-19 pandemic, ACE2 has gained attention in the scientific community (38). ACE2 acts as viral receptor for COVID-19. The binding of the virus leads to ACE2 entry into the cell rendering the enzyme unavailable to counter the ACE effect (39). The imbalance between Ang-(1-7) and Ang II is associated with disease severity and seems to play a role in ARDS commonly seen in severe COVID-19 infection (40,41).
Work up for hypertension
It may seem difficult to decide which child to work up for hypertension. Although the prevalence of inherited hypertension is unknown and thought to be rare, there are increasing numbers of reports about patients with inherited hypertension (42) (Table 2). With this manuscript, we attempt to increase the sensitivity and suspicion of the general provider. The level of scrutiny may be low as children with inherited forms of hypertension may initially present without severe hypertension and the associated electrolyte disorders may be absent. Work-up of hypertension starts with a good history and physical exam. The history should focus on prematurity, pain, medications, snoring (as obstructive sleep apnea also contributes to hypertension), and very importantly the family history. The family history should not only include kidney disease, hypertension, and need for a kidney transplant but also myocardial infarctions, aortic aneurysms, and strokes (16). In our opinion, an adolescent patient with a stroke and a positive family history of hypertension should undergo a thorough work-up.
Laboratory work up should include a urinalysis, chemistry panel including electrolytes, BUN, and creatinine, lipid profile (fasting), HgbA1c, liver enzymes, TSH, T4, cortisol (morning level), renin, aldosterone, and CBC (11,24). The hyperkalemia linked with Gordon syndrome is not always present (43). Hypokalemia is only diagnosed in 50% of glucocorticoid-remediable aldosteronism (GRA) (44) and is not persistently diagnosed in all patients with apparent mineralocorticoid excess (AME) (45) or Liddle’s syndrome (46). In most of the inherited forms of hypertension mild metabolic alkalosis is detected except for Gordon syndrome which usually is associated with metabolic acidosis. Urine electrolytes are not helpful in the diagnosis. Perhaps the most useful tools in the detection of inherited forms of hypertension are the renin and aldosterone levels (47). Preferably, these parameters should be obtained prior to therapy, in particular when an ACE inhibitor or ARB is used. A helpful tool to assess for increased aldosterone activity is the plasma aldosterone (ng/dL)-to-plasma renin activity (ng/mL/h) ratio, which is an indicator for excess aldosterone even in the context of a normal plasma aldosterone level. An aldosterone-to-renin ratio above 30 is consistent with primary aldosteronism (PA) (48). Additional tests may include a drug screen if there is suspicion for substance abuse. A renal ultrasound may detect cystic kidney disease or a renal mass.
Hyporeninemic hypertension
These patients constitute a group of individuals who have been excluded to have common causes of hypertension such as renovascular hypertension, kidney disease, coarctation of the aorta, pheochromocytoma, or hyperthyroidism (Table 1). In the past, the cause for the suspected hypertension remained unclear but with improved genetic testing inherited causes of hypertension have become better understood and are often characterized by hyporeninemia. While idiopathic or primary hypertension is often thought to be polygenic in origin hyporeninemic hypertension is frequently inherited due to a single gene mutation in a Mendelian fashion (48-50). Progress made in molecular genetics over the last 40 years resulted in the identification of a number of hyporeninemic conditions which have improved our understanding of the pathophysiology and have allowed for the development of targeted treatments. Some of these initially considered rare conditions have proven to be more common and should be considered in hypertensive children (15), especially given a positive family history of hypertension, stroke, or end-stage kidney disease. A typical feature of these inherited forms of hypertension is a suppressed low renin level due to expanded extracellular volume as a consequence from inappropriate Na+ absorption in the distal nephron. This is consistent with the hypothesis that dysfunctional mechanisms to regulate Na+ and volume balance due to impaired renal natriuresis is part of the pathomechanism of hypertension (51,52).
The different modalities of Na+ transport in the distal nephron
At the root of many forms of hyporeninemic hypertension is overstimulation of Na+ absorption in the distal nephron. There are two sites for Na+ absorption in the distal nephron, the distal convoluted tubule (DCT) and the collecting duct (CD) (48). In the DCT Na+ enters the apical thiazide-sensitive sodium-chloride-cotransporter (NCC) along its concentration gradient into the cell (Figure 1). In the CD Na+ enters the cytosol via the apical amiloride-sensitive epithelial Na+ channel (ENaC) (Figure 2). On the basolateral side of these cells Na+ is extruded by the Na+/K+-ATPase. The Na+ absorption from the luminal side is not only crucial to maintain Na+ and fluid absorption but also to maintain the lumen-negative transmembrane potential which affects the activity of other ion channels and provides the driving force for potassium (K+) and hydrogen (H+) secretion (48). This is how excess Na+ absorption in the DCT and CD contribute to hypokalemia and metabolic alkalosis.
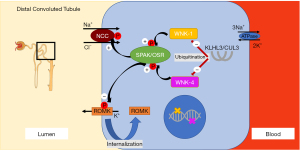
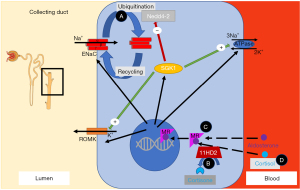
Different mechanisms regulate Na+ absorption in the distal nephron. Aldosterone binds to the mineralocorticoid receptor (MR), which then enhances Na+ absorption via NCC and ENaC (Figure 2) (53). Stimulation of the MR activates genomic and non-genomic signaling pathways including the serum- and glucocorticoid-inducible protein kinase 1 (SGK1) to enhance Na+ absorption in the distal nephron (Figure 2) (54). However, the MR cannot distinguish between aldosterone and cortisol. The cytoplasmic enzyme 11β-hydroxysteroid dehydrogenase type 2 enzyme (11β-HSD2) converts cortisol to inactive metabolites and thus prevents the MR from cortisol oversaturation (Figure 2). This is significant as cortisol is highly abundant, whereas aldosterone is much less present. The aldosterone to cortisol ratio in circulation is approximately 1:100 to 1:1,000 while the MR has a similar binding affinity for aldosterone and cortisol (55). Other modifiers for distal Na+ and K+ transport are two “with no lysine (K) serine/threonine protein kinases” (WNKs) called WNK-1 and WNK-4 which are mutated in pseudohypoaldosteronism type II (PHAII), a form of hyporeninemic hypertension (Figure 1) (56-58). WNK-1 and WNK-4 indirectly modify NCC activity by stimulating STE-20 serine proline alanine-rich kinase (SPAK) and oxidative stress response 1 kinase (OSR1) which in turn stimulates NCC phosphorylation (Figure 1) (59-62). WNK-1 also stimulates ENaC via SGK1 (not shown in Figure 2) (63). WNK-1 and WNK-4 mutations are thought to increase WNK-1 and WNK-4 gene expression, their activities on Na+ absorption and thereby cause volume overload (59,64). The identification of mutations in the genes for Cullin 3 (CUL3) and Kelch-like 3 (KLHL3) in patients with PHAII provided further insight in the regulation of WNKs (65). CUL3 and KLHL3 form a RING-type ubiquitin ligase complex, which promotes proteasomal degradation of WNKs (Figure 1) (66). Patients with CUL3 and KLHL3 mutations fail to degrade WNKs resulting in higher WNK activity and Na+ absorption (67,68). PHAII patients with CUL3 and KLHL3 mutations have a more severe phenotype than patients with WNK-1 and WNK-4 mutations (65). The regulation of Na+ transport remains a topic of investigation. Below we only discuss genes known to contribute to upregulated Na+ transport and hypertension. We will divide the diseases into disorders of the distal nephron (with mostly low aldosterone) and primary adrenal diseases (mostly high aldosterone) (Figure 3).

Distal nephron disorders (hyporeninemic-low aldosterone hypertension)
Liddle syndrome
This is an autosomal dominant disorder caused by the increased activity of ENaC in the principal cells located in the distal nephrons (69). This condition was first described by Grant Liddle in 1963 in a family with multiple members developing severe hypertension and hypokalemia (70). Liddle coined the term pseudoaldosteronism because the clinical presentation resembled hyperaldosteronism, but aldosterone levels were suppressed (70). ENaC is an obligate heterotrimer consisting of an α, β, and γ subunit. In the kidney, ENaC is primarily expressed in the apical membranes of the late DCT, connecting tubule, and CD. Under physiologic conditions, ENaC is regulated by aldosterone which upregulates the expression of ENaC channel via the MR in principle cells (Figure 2) (71). Patients with Liddle syndrome have mutations in the genes encoding the β or γ subunits of ENaC (72,73). Both subunits facilitate the binding of Nedd4, a factor that promotes ENaC internalization and degradation. The lack of Nedd4 binding may impair ENaC retrieval and increases the number of functional ENaC channels at the apical surface of principal cells (74,75). Subsequently, this leads to increased Na+ absorption, plasma volume expansion and hypertension (76). Additional studies also showed that truncation of the ENaC β subunit resulted in increased cell surface channel expression and channel open probability (77). Other mechanisms how certain ENaC mutations cause channel overactivity include insensitivity of ENaC to high intracellular Na+ concentration, changes of ENaC by proteolytic cleavage, channel open probability, and non-NEDD 4-2 mediated modifications of channel trafficking (75,78-81).
Liddle syndrome is considered the most common type of monogenic hypertension (48). Hypertension often starts in childhood but may be asymptomatic and remains undiagnosed. Laboratory studies show low plasma renin activity and aldosterone along with hypokalemia and metabolic alkalosis. Hypokalemia and metabolic alkalosis develop in response to increased Na+ reabsorption which creates a negatively charged lumen which promotes secretion of K+ and H+ ions into the lumen (82). Treatment for Liddle syndrome consists of a low salt diet and inhibition of ENaC (83). Amiloride or triamterene directly interfere with ENaC, lower effectively blood pressure, and correct the hypokalemia and alkalosis in Liddle Syndrome. Both agents block the constitutively active ENaC and thus prevent excessive Na+ reabsorption (84). Untreated Liddle syndrome patients are at grave risk for cardiovascular morbidity and mortality including strokes (84).
Gordon’s syndrome (PHAII)
This condition is also known as PHAII and caused by increased activity of NCC in the distal nephron (85). This condition was first characterized by Richard Gordon in several Australian families in 1970 (86). Because PHAII patients responded well to low-dose thiazide therapy, this condition has been associated with abnormal Na+ absorption and enhanced NCC function long before the causative genes were identified (87). Although, it was well known that patients with Gordon syndrome have increased Na+ reabsorption in the DCT, the molecular mechanism of increased NCC activity was only discovered over the last twenty years by Richard Lifton’s group (56,65). His group identified autosomal dominant mutations, which increased the activity of WNK-1 and WNK-4 (Figure 1) (56,65). Both kinases increase the activity of NCC and increase the internalization of the renal outer medullary potassium (ROMK) channel which secrets K+, which contributes to hyperkalemia (88,89). Moreover, there are different characteristics attributed to a shorter kidney-specific and a more ubiquitous, longer version of WNK-1 (90). Large deletions within the first intron of the WNK-1 gene result in increased WNK-1 expression. Missense mutations in WNK-4 interfere with endosomal degradation of WNK-4. Both kinases phosphorylate SPAK/OSR, which in turn phosphorylates NCC resulting in increased NCC activity (Figure 1). Moreover, WNK-1 also stimulates ENaC via SGK1 (63). Other genes causing Gordon syndrome or PHAII include KLHL3 and CUL3 (65). Both proteins are part of a ubiquitin-protein ligase complex, which degrades WNKs (56,59,65). The presentation of patients with Gordon Syndrome/PHAII is quite variable. The patients are characterized by short stature, hypertension, hyperkalemia, muscle weakness, dental abnormalities, and intellectual developmental delay (56,65,91). The hyperkalemia and metabolic acidosis frequently precede the presentation of hypertension, and hypertension may only be diagnosed later in adult life (92,93). The initial presentation can sometimes be reminiscent of renal tubular acidosis (RTA) type 4 but in contrast to many forms of RTA type 4 glomerular filtration rate is usually normal in PHAII/Gordon syndrome (92). Patients with CUL3 and KLHL3 mutations usually have a more serious phenotype compared to patients with WNK-1 and WNK-4 mutations (65). Laboratory studies show suppressed plasma renin activity and normal or elevated aldosterone along with hyperkalemia and metabolic acidosis. The elevated aldosterone level may be due to direct activation of aldosterone due to hyperkalemia. The hyperkalemia seems to be due to a combination of increased ROMK internalization with less K+ secretion and due to decreased Na+ delivery to distal nephron (85,94). Interestingly, many PHAII/Gordon syndrome patients also display hypercalciuria. Treatment for these patients includes thiazides along with Na+ and K+ restriction (87).
Apparent mineralocorticoid excess (AME)
This is a rare autosomal recessive disorder characterized by inactivating mutations of the HSD11B2 gene, which encodes the 11β-HSD2 enzyme (95). This condition was first reported by Werder et al. in 1974 (96). The 11β-HSD2 enzyme converts cortisol to cortisone. As outlined above this conversion is critical to prevent overstimulation of the MR by cortisol because cortisone has 100-fold less binding affinity to MRs. The mutation interferes with the proper conversion of cortisol to cortisone and results in higher cortisol levels, which then bind to the MR and result in overstimulation of Na+ absorption in the distal nephron (97). Characteristics of AME are unregulated activation of Na+ reabsorption, K+ secretion, and H+ secretion. These patients commonly develop end-organ damage in the heart, the central nervous system, the kidneys, and the retina, and the mortality in undiagnosed patients is considered to be high (98,99). Initially, it was thought, that this condition always presents in childhood with a grave phenotype including failure to thrive, low weight birth, and hypokalemic metabolic alkalosis. Later, milder phenotypes have been published with development of hypertension in adulthood and without any electrolyte abnormalities (100-102). These milder cases are due to only partial inactivation of 11β-HSD2 (101,102). Patients can also present with a urinary concentration defect most likely caused by the chronic hypokalemia, which contributes to diabetes insipidus. Sometimes also hypercalciuria and nephrocalcinosis can be seen which are unclear in their etiology (101). AME should be considered in any patient with hyporeninemic, low aldosterone hypertension and indications of mineralocorticoid excess. The lack of 11β-HSD2 activity can be detected in the urine with an abnormal ratio of cortisol metabolites [for example tetrahydrocortisol (THF)] and allotetrahydrocortisol (5αTHF) to cortisone metabolites such as tetrahydrocortisone (THE) (103). A urine steroid profile showing the ratio of THF + 5αTHF to THE in a 24 h urine can be diagnostic. In patients with a milder clinical course of AME the urine steroid profile can be normal requiring genetic testing. Treatment includes dietary Na+ restriction, use of MR antagonists (e.g., spironolactone and the more specific MR antagonist eplerenone), and K+ supplementation. Addition of amiloride may help to conserve K+. In case of hypercalciuria or nephrocalcinosis thiazides have been used. It is important to distinguish this condition from acquired suppression of 11β-HSD2 activity by liquorice (104).
Hypertension exacerbated by pregnancy (Geller syndrome)
The name of this condition is misleading because this disease is not limited to females (105). Affected patients have an autosomal dominant, activating mutation in the gene NR3C2, which encodes the MR. The activating mutation results in a constitutively active MR and overstimulation of Na+ absorption not only by aldosterone but also cortisone, and progesterone (105). While hypertension is present and possibly severe in nonpregnant patients, the hypertension is worsening during pregnancy. This condition can manifest at an early age and is accompanied by symptoms of mineralocorticoid excess. It is thought that the mutation increases the sensitivity of the MR to non-mineralocorticoid steroids, for example progesterone, which increases about 100-fold during pregnancy. Interestingly, the mutated form of the MR can also be stimulated by spironolactone. Laboratory studies show low renin, and aldosterone, and normal or low K+. Treatment includes salt restriction, thiazides, or ENaC antagonists. MR antagonists should be avoided.
Distal nephron disorders (hyporeninemic-low aldosterone hypertension
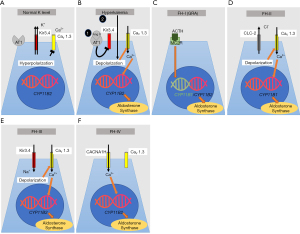
Under physiologic conditions, aldosterone synthesis is regulated by calcium influx through voltage-gated Ca2+ channels (CaV1.3) located at the apical surface of adrenal granulosa cells (Figure 4A) (106). At baseline, granulosa cells are hyperpolarized due to a high resting K+ conductance with K+ leaving the cells via inward rectifier potassium channel (Kir3.4). Inhibition of Kir3.4 either by binding of Ang II to angiotensin II receptors and/or by high serum potassium results in membrane depolarization (Figure 4B). This opens voltage-gated Ca2+ channels and results in Ca2+ influx which stimulates aldosterone synthase expression (106). Excessive aldosterone production can be primary due to bilateral adrenal hyperplasia or a unilateral adrenal adenoma (22,107). Even serum aldosterone levels within the reference range are capable of affecting blood pressure adversely (108). The aldosterone-to-renin ratio is a good indicator of aldosterone excess and is associated with hypertension, even in patients with normal aldosterone (22). Aldosterone and the RAAS may be rapidly stimulated due to hypotension and hypovolemia, which is mediated through adrenal aldosterone synthesis, intermediate-term activation may interfere with salt homeostasis, and long-term activation which results in structural changes of end-organs.
Primary aldosteronism (PA)
This condition is commonly due to either an aldosterone-producing adrenal adenoma or bilateral adrenal hyperplasia and is one of the most common causes of secondary hypertension (109). Compared with patients affected by essential hypertension, patients with PA have an increased risk of cardiovascular disease (110,111). A higher risk for myocardial infarction, atrial fibrillation, kidney disease, and stroke has been persistently published in PA patients (111-114). PA is diagnosed in about 5% of hypertensive patients in a primary care setting. However, with increasing severity of hypertension this prevalence increases up to 20% (115,116). One report estimates that PA affects 8% and 13% of individuals with hypertension grades 2 and 3, respectively (117). Sporadic forms of hyperaldosteronism are common, familial forms are much less frequent with approximately 6% (118). The recommended screening test for both familial and non-familial forms of primary hyperaldosteronism is the ratio of plasma aldosterone concentration to plasma renin activity. A ratio higher than 30 is widely accepted as a positive screening-test result. However, normative values for the aldosterone-to-renin ratio are not well established for the pediatric population (119). The degree of aldosterone secretion and the aldosterone-to-renin ratio correlate with severity of hypertension (117). Confirmation of the diagnosis is dependent upon measurement of urinary aldosterone in a 24 h urine collection and an aldosterone suppression test applying oral Na+, IV saline, fludrocortisone, or captopril. The gold standard to diagnose PA is adrenal venous sampling which is mostly performed in adults (120). In patients with a family history of hypertension who present with hypertension and hypokalemia, one should consider screening for genetic causes of primary hyperaldosteronism. Next, we will outline a number or genetically inherited forms of hyperaldosteronism.
Familial hyperaldosteronism (FH)
This group includes a number of autosomal dominant, rare conditions. They usually present with early-onset hypertension, hypokalemia, metabolic alkalosis, and high aldosterone-to-renin ratio (15). Four different types of FH are known, and it can be challenging to distinguish them from sporadic PA based on clinical findings and biochemical tests (109).
Familial hyperaldosteronism type I (FH-1 or glucocorticoid-remediable aldosteronism)
FH-I—also known as GRA—was first described in 1966 by Sutherland et al. and is responsible for <1% of all PA patients (121,122). FH-I/GRA is an autosomal dominant condition and was the first form of monogenic hypertension to be recognized as a single-gene hypertensive disorder (123). Aldosterone is synthesized by aldosterone synthase in the zona glomerulosa of the adrenal cortex under the control of angiotensin II. Cortisol is produced by 11β-hydroxylase in the zona fasciculata under the control of the adrenocorticotropic hormone (ACTH). The genes encoding aldosterone synthase and 11β-hydroxylase are located adjacent to each other on chromosome 8. Lifton et al. identified that FH-1/GRA is due to a hybrid/chimeric gene on chromosome 8q consisting of the regulatory region of the 11β-hydroxylase gene, CYP11B1, coupled with the structural region of the aldosterone synthase gene, CYP11B2, caused by asymmetrical cross-over (123). This means that the hybrid gene contains an ACTH-responsive promoter and an aldosterone synthase encoding region which results in ACTH-dependent stimulation and is independent of renin, angiotensin, K+ or Na+ balance (Figure 4C) (123-125). Patients with this condition present with hypertension in infancy or childhood and are characterized by profound cardiovascular morbidity and an increased risk for thoracoabdominal aneurysms and hemorrhagic strokes due to ruptured aneurysms (126-129). Therefore, all patients with genetically proven FH-I/GRA should undergo screening with cerebral MR angiogram at puberty, and subsequently every 5 years (107,130). However, also milder forms and even normotensive patients with GRA have been published (126,131,132). Some patients were only diagnosed in adolescence (133). Hypokalemia is only present in about 50% of GRA patients and some also display mild metabolic alkalosis (126,131,133). The pattern of crossovers are different in each pedigree suggesting that the mutations occurred independently of each other in each kindred (131). GRA seems to be much less prevalent in African-Americans. Plasma renin activity is typically suppressed while aldosterone levels can be increased (aldosterone-to-renin ratio >30, normal is <20). In some patients the aldosterone may be normal and only the aldosterone-to-renin ratio may be elevated. Other diagnostic tools include a dexamethasone suppression test, urinary steroid profile (with an elevated 18-oxocortisol level), adrenal imaging, and adrenal vein sampling. Improved genetic testing capabilities have eliminated the need for challenging studies and sampling. Preferred treatment of GRA consists of low-dose glucocorticoids to downregulate ACTH-stimulated mineralocorticoid production, amiloride, and spironolactone, which blocks binding of aldosterone to the MR. To treat hypertension due to GRA the steroid dose is usually not very high dose. Thiazide diuretics are not the recommended first-line treatment, but might improve control of blood pressure when used concurrently with spironolactone. Thiazide diuretics can cause marked hypokalemia secondary to increased Na+ delivery to the cortical collecting duct.
Familial hyperaldosteronism type II (FH-II)
Initially described by Stowasser et al. in 1992, 13 patients from five families were found to have PA, which was not suppressed by a dexamethasone challenge (distinguishing it from FH-I/GRA). FH-II is now recognized as another autosomal dominant cause of secondary hypertension (134). A genetic locus for FH-II has been described on chromosome 7p22 (135). Dominant gain-of-function mutations of the CLCN2 gene were identified (136,137). FH-II has a prevalence between 1.2% and 6% in adults with PA (109). This gene encodes the voltage-gated chloride channel ClC-2 in the adrenal zona glomerulosa. Mutations result in a lower threshold for membrane depolarization in the adrenal zona glomerulosa and allow for stimulation of voltage-gated calcium channels. Consequently, the higher calcium influx upregulates CYP11B2 expression, which encodes aldosterone synthase (Figure 4D) (136). A mouse model for FH-II with one of the most common human missense mutations in CLCN2 reproduced the human phenotype and confirmed the role of ClC-2 in aldosterone synthesis (138). Patients with FH-II are clinically indistinguishable from those with sporadic forms of PA due to bilateral adrenal hyperplasia. FH-II patients frequently have a family history of adrenal hyperplasia or adenoma. Given extended inclusion criteria FH-II seems to be more common than previously considered and may be the most common cause of inherited hypertension in adults (139). In contrast to FH-I/GRA, FH-II patients usually present in adolescence and in adulthood while irregularities of the RAAS may be detected earlier (140). FH-II patients can also develop aldosterone-producing adenoma or bilateral adrenal hyperplasia. It is important to differentiate between FH-I and FH-II as they require different treatments. Direct genetic testing for the presence of the chimeric gene in FH-I/GRA is available and has been shown to have 100% sensitivity and specificity for diagnosing FH-I/GRA (141). FH-I/GRA patients are treated with glucocorticoids to decrease ACTH driven overproduction of aldosterone. By contrast, hypertension in FH-II is unresponsive to glucocorticoids, but MR antagonists and adrenalectomy are effective.
Familial hyperaldosteronism type III (FH-III)
This is another autosomal dominant rare form of monogenic hypertension accounting for approximately 0.3% of PA patients (142,143). The causative gene for FH-III is KCNJ5 which encodes the inwardly rectifying K+ channel Kir3.4, also named GIRK4 (144). KCNJ5 mutations cause both familial and sporadic forms of primary hyperaldosteronism (144,145). KCNJ5 somatic mutations in the adrenal gland are also responsible for 40% of aldosterone producing adenomas (144,146). As a consequence of the KCNJ5 mutation the selectivity of the Kir3.4 pore is lost and a higher influx of Na+ occurs through Kir3.4 (Figure 4E). This results in membrane depolarization, calcium influx and increased CYP11B2 expression, encoding for aldosterone synthase (147). Patients can present with bilateral adrenal hyperplasia, severe hypertension, and hypokalemia. While most patients with FH-III are adults, a few pediatric patients with FH-III are also published (145,148-151). Genetic testing is very helpful to make the correct diagnosis. There should be a low threshold for adrenal computed tomography and adrenal venous sampling. Follow-up may include repeated imaging of the adrenal glands to exclude development of a mass that requires surgical resection. Some mutations are not germline mutations but only somatic KCNJ5 mutations, and will only be detected in tissue from the adrenal gland (144).
Familial hyperaldosteronism type IV (FH-IV)
This type of familial hyperaldosteronism is caused by autosomal dominant germline mutations in CACNA1H. This gene encodes for the α subunit of the calcium channel Cav3.2 which is highly abundant in the zona glomerulosa of the adrenal gland (Figure 4F) (152). Gain-of-function mutations in CACNA1H increase calcium influx and increased aldosterone production (153). The symptoms are comparable to other forms of FH. Genetic testing is recommended for patients younger than 10 years of age with PA and hypertension (154). Specific treatment is not available for patients with this condition but mineralocorticoid antagonists or adrenalectomy improve hypertension (152).
Other genetic forms of FH also include either somatic and/or germline mutations in CACNA1D, ATP1A1, ATP2B3, and PRKACA causing PA and aldosterone-producing adenomas (155-158).
Congenital adrenal hyperplasia
The term ‘congenital adrenal hyperplasia’ (CAH) describes a group of syndromes caused by defects in cortisol biosynthesis. CAH is inherited in an autosomal recessive fashion. CAH is caused by a variety of different enzymatic defects. When 21-hydroxylase (CYP21A2) is deficient—the most common cause of CAH—patients are usually not hypertensive. In 11β-hydroxylase (CYP11B1) and 17α-hydroxylase (CYP17) deficiencies, patients present with hypertension and hypokalemia due to the overproduction of ACTH, 21-hydroxylated mineralocorticoids, including deoxycorticosterone (DOC), which has mineralocorticoid activity and stimulates the MR (159,160). The patients develop hypertension due to Na+ retention mediated by the distal nephron. Moreover, defects in CYP11B1 and CYP17 inhibit cortisol synthesis with a subsequent reduction in feedback inhibition of ACTH secretion. Consequently, the increased ACTH secretion stimulates production of steroid precursors proximal to the ‘blocked’ enzymatic step, leading to excessive levels of DOC. In both disorders, patients present with hypertension and hypokalemia early in life. Signs of androgen excess distinguish the two disorders; 11β-hydroxylase deficiency causes virilization in girls and precocious puberty in boys, whereas 17α-hydroxylase deficiency results in sex hormone deficiency, primary amenorrhea and delayed sexual development in girls, and ambiguous genitalia in boys. The correct diagnosis is made by the clinical presentation and plasma or urine steroid profiles. Genetic diagnosis of both conditions relies on testing for mutations that either severely depress or abolish enzyme activity. The hypertension can be addressed with MR antagonists. Both conditions are treated with exogenous glucocorticoids, which normalize ACTH secretion and ACTH-mediated build-up of cortisol precursors proximal to the enzymatic deficiency, including DOC. Acquired forms of CAH caused by DOC-producing tumors typically present later in life.
Familial glucocorticoid resistance
Individuals with mutations in the glucocorticoid receptor (GR) do not respond to cortisol and lack the subsequent suppression of cortisol synthesis. Due to the GR unresponsiveness affected patients develop overproduction of cortisol and androgens (161). The elevated cortisol levels then overwhelm the capability of the 11β-HSD2 to convert it to cortisone. This results in MR activation and excessive Na+ absorption. Given the GR resistance to cortisol patients do not develop cushingoid symptoms. The inheritance depends on the specific mutation and the degree of symptoms and hypertension depend on the severity of GR impairment (162). Complete GR inactivation is incompatible with life. The diagnosis is made with significantly elevated plasma cortisol levels. The hypertension is treated with MR antagonists.
Acknowledgments
Funding: Matthias T. F. Wolf is supported by Children’s Clinical Research Advisory Committee (CCRAC), Department of Defense (W81XWH1910205), and the NIH (P30 DK079328-11, R01DK119631).
Footnote
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-48/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-48/coif). MTFW reports research funding from the Department of Defense (W81XWH1910205), the National Institute of Health (P30 DK079328-11, R01DK119631), and the Children’s Clinical Research Advisory Committee (CCRAC). The other author has no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Writing Group Members. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016;133:447-54. [Crossref] [PubMed]
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281-357. [Crossref] [PubMed]
- Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347-60. [Crossref] [PubMed]
- Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health (Abingdon) 2003;16:230. [PubMed]
- Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases--where worlds meet. N Engl J Med 2010;363:1196-8. [Crossref] [PubMed]
- Islam MS. Hypertension: From Basic Research to Clinical Practice. Adv Exp Med Biol 2017;956:1-2. [Crossref] [PubMed]
- Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 2008;117:3171-80. [Crossref] [PubMed]
- Son JS, Choi S, Kim K, et al. Association of Blood Pressure Classification in Korean Young Adults According to the 2017 American College of Cardiology/American Heart Association Guidelines With Subsequent Cardiovascular Disease Events. JAMA 2018;320:1783-92. [Crossref] [PubMed]
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20. [Crossref] [PubMed]
- The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555-76. [Crossref] [PubMed]
- Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017;140:e20171904. [Crossref] [PubMed]
- Chiolero A, Cachat F, Burnier M, et al. Prevalence of hypertension in schoolchildren based on repeated measurements and association with overweight. J Hypertens 2007;25:2209-17. [Crossref] [PubMed]
- McNiece KL, Poffenbarger TS, Turner JL, et al. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr 2007;150:640-4, 644.e1.
- Skinner AC, Perrin EM, Moss LA, et al. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med 2015;373:1307-17. [Crossref] [PubMed]
- Lu YT, Fan P, Zhang D, et al. Overview of Monogenic Forms of Hypertension Combined With Hypokalemia. Front Pediatr 2020;8:543309. [Crossref] [PubMed]
- McCrindle BW. Assessment and management of hypertension in children and adolescents. Nat Rev Cardiol 2010;7:155-63. [Crossref] [PubMed]
- Bühler FR, Bolli P, Kiowski W, et al. Renin profiling to select antihypertensive baseline drugs. Renin inhibitors for high-renin and calcium entry blockers for low-renin patients. Am J Med 1984;77:36-42. [PubMed]
- Burrello J, Monticone S, Buffolo F, et al. Is There a Role for Genomics in the Management of Hypertension? Int J Mol Sci 2017;18:1131. [Crossref] [PubMed]
- Garovic VD, Hilliard AA, Turner ST. Monogenic forms of low-renin hypertension. Nat Clin Pract Nephrol 2006;2:624-30. [Crossref] [PubMed]
- Mulatero P, Verhovez A, Morello F, et al. Diagnosis and treatment of low-renin hypertension. Clin Endocrinol (Oxf) 2007;67:324-34. [Crossref] [PubMed]
- Ahn SY, Gupta C. Genetic Programming of Hypertension. Front Pediatr 2017;5:285. [Crossref] [PubMed]
- Tomaschitz A, Pilz S, Ritz E, et al. Aldosterone and arterial hypertension. Nat Rev Endocrinol 2010;6:83-93. [Crossref] [PubMed]
- Sparks MA, Crowley SD, Gurley SB, et al. Classical Renin-Angiotensin system in kidney physiology. Compr Physiol 2014;4:1201-28. [Crossref] [PubMed]
- Brady TM. Hypertension. Pediatr Rev 2012;33:541-52. [Crossref] [PubMed]
- Simões E, Silva AC. Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res 2016;107:154-62. [Crossref] [PubMed]
- Forrester SJ, Booz GW, Sigmund CD, et al. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev 2018;98:1627-738. [Crossref] [PubMed]
- Rocha NP, Simoes E, Silva AC, Prestes TRR, et al. RAS in the Central Nervous System: Potential Role in Neuropsychiatric Disorders. Curr Med Chem 2018;25:3333-52. [Crossref] [PubMed]
- Shenoy V, Ferreira AJ, Qi Y, et al. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med 2010;182:1065-72. [Crossref] [PubMed]
- Simões E, Silva AC, Miranda AS, Rocha NP, et al. Renin angiotensin system in liver diseases: Friend or foe? World J Gastroenterol 2017;23:3396-406. [Crossref] [PubMed]
- Patel S, Rauf A, Khan H, et al. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother 2017;94:317-25. [Crossref] [PubMed]
- Simões E, Silva AC, Flynn JT. The renin-angiotensin-aldosterone system in 2011: role in hypertension and chronic kidney disease. Pediatr Nephrol 2012;27:1835-45. [Crossref] [PubMed]
- Bader M. Cardiovascular Hormone Systems: From Molecular Mechanisms to Novel Therapeutics. Hoboken, New Jersey: John Wiley & Sons, 2008.
- Simões E, Silva AC, Lanza K, Palmeira VA, et al. 2020 update on the renin-angiotensin-aldosterone system in pediatric kidney disease and its interactions with coronavirus. Pediatr Nephrol 2021;36:1407-26. [Crossref] [PubMed]
- Beldent V, Michaud A, Wei L, et al. Proteolytic release of human angiotensin-converting enzyme. Localization of the cleavage site. J Biol Chem 1993;268:26428-34. [Crossref] [PubMed]
- Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87:E1-9. [Crossref] [PubMed]
- Schiavone MT, Santos RA, Brosnihan KB, et al. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1-7) heptapeptide. Proc Natl Acad Sci U S A 1988;85:4095-8. [Crossref] [PubMed]
- Patel VB, Zhong JC, Grant MB, et al. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res 2016;118:1313-26. [Crossref] [PubMed]
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. [Crossref] [PubMed]
- Lanza K, Perez LG, Costa LB, et al. Covid-19: the renin-angiotensin system imbalance hypothesis. Clin Sci (Lond) 2020;134:1259-64. [Crossref] [PubMed]
- Rodrigues Prestes TR, Rocha NP, Miranda AS, et al. The Anti-Inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Curr Drug Targets 2017;18:1301-13. [Crossref] [PubMed]
- Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020;201:1299-300. [Crossref] [PubMed]
- Raina R, Krishnappa V, Das A, et al. Overview of Monogenic or Mendelian Forms of Hypertension. Front Pediatr 2019;7:263. [Crossref] [PubMed]
- Disse-Nicodème S, Achard JM, Potier J, et al. Familial hyperkalemic hypertension (Gordon syndrome): evidence for phenotypic variability in a study of 7 families. Adv Nephrol Necker Hosp 2001;31:55-68. [PubMed]
- Litchfield WR, Coolidge C, Silva P, et al. Impaired potassium-stimulated aldosterone production: a possible explanation for normokalemic glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 1997;82:1507-10. [Crossref] [PubMed]
- Tapia-Castillo A, Baudrand R, Vaidya A, et al. Clinical, Biochemical, and Genetic Characteristics of "Nonclassic" Apparent Mineralocorticoid Excess Syndrome. J Clin Endocrinol Metab 2019;104:595-603. [Crossref] [PubMed]
- Cui Y, Tong A, Jiang J, et al. Liddle syndrome: clinical and genetic profiles. J Clin Hypertens (Greenwich) 2017;19:524-9. [Crossref] [PubMed]
- Hundemer GL, Vaidya A. Primary Aldosteronism Diagnosis and Management: A Clinical Approach. Endocrinol Metab Clin North Am 2019;48:681-700. [Crossref] [PubMed]
- Vehaskari VM. Heritable forms of hypertension. Pediatr Nephrol 2009;24:1929-37. [Crossref] [PubMed]
- Deng AY. Genetic mechanisms of polygenic hypertension: fundamental insights from experimental models. J Hypertens 2015;33:669-80; discussion 680. [Crossref] [PubMed]
- Deng AY. Genetic basis of polygenic hypertension. Hum Mol Genet 2007;16 Spec No. 2:R195-202.
- Guyton AC, Coleman TG, Cowley AV Jr, et al. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 1972;52:584-94. [Crossref] [PubMed]
- Hall JE, Mizelle HL, Hildebrandt DA, et al. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension 1990;15:547-59. [Crossref] [PubMed]
- Kim GH, Masilamani S, Turner R, et al. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 1998;95:14552-7. [Crossref] [PubMed]
- Helms MN, Fejes-Toth G, Naray-Fejes-Toth A. Hormone-regulated transepithelial Na+ transport in mammalian CCD cells requires SGK1 expression. Am J Physiol Renal Physiol 2003;284:F480-7. [Crossref] [PubMed]
- Zennaro MC, Boulkroun S, Fernandes-Rosa F. Inherited forms of mineralocorticoid hypertension. Best Pract Res Clin Endocrinol Metab 2015;29:633-45. [Crossref] [PubMed]
- Wilson FH, Disse-Nicodème S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science 2001;293:1107-12. [Crossref] [PubMed]
- Wilson FH, Kahle KT, Sabath E, et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci U S A 2003;100:680-4. [Crossref] [PubMed]
- Yang CL, Angell J, Mitchell R, et al. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 2003;111:1039-45. [Crossref] [PubMed]
- Ostrosky-Frid M, Castañeda-Bueno M, Gamba G. Regulation of the renal NaCl cotransporter by the WNK/SPAK pathway: lessons learned from genetically altered animals. Am J Physiol Renal Physiol 2019;316:F146-58. [Crossref] [PubMed]
- Gamba G. Regulation of the renal Na+-Cl- cotransporter by phosphorylation and ubiquitylation. Am J Physiol Renal Physiol 2012;303:F1573-83. [Crossref] [PubMed]
- Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, et al. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A 2012;109:7929-34. [Crossref] [PubMed]
- Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, et al. The Effect of WNK4 on the Na+-Cl- Cotransporter Is Modulated by Intracellular Chloride. J Am Soc Nephrol 2015;26:1781-6. [Crossref] [PubMed]
- Xu BE, Stippec S, Chu PY, et al. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci U S A 2005;102:10315-20. [Crossref] [PubMed]
- Hadchouel J, Ellison DH, Gamba G. Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases. Annu Rev Physiol 2016;78:367-89. [Crossref] [PubMed]
- Boyden LM, Choi M, Choate KA, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 2012;482:98-102. [Crossref] [PubMed]
- Shibata S, Zhang J, Puthumana J, et al. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A 2013;110:7838-43. [Crossref] [PubMed]
- Wakabayashi M, Mori T, Isobe K, et al. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep 2013;3:858-68. [Crossref] [PubMed]
- Wu G, Peng JB. Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation. FEBS Lett 2013;587:1717-22. [Crossref] [PubMed]
- Hansson JH, Nelson-Williams C, Suzuki H, et al. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 1995;11:76-82. [Crossref] [PubMed]
- Liddle GW. A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Phys 1963;76:199-213.
- Soundararajan R, Pearce D, Ziera T. The role of the ENaC-regulatory complex in aldosterone-mediated sodium transport. Mol Cell Endocrinol 2012;350:242-7. [Crossref] [PubMed]
- Shimkets RA, Warnock DG, Bositis CM, et al. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 1994;79:407-14. [Crossref] [PubMed]
- Hiltunen TP, Hannila-Handelberg T, Petäjäniemi N, et al. Liddle's syndrome associated with a point mutation in the extracellular domain of the epithelial sodium channel gamma subunit. J Hypertens 2002;20:2383-90. [Crossref] [PubMed]
- Staub O, Dho S, Henry P, et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J 1996;15:2371-80. [Crossref] [PubMed]
- Knight KK, Olson DR, Zhou R, et al. Liddle's syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci U S A 2006;103:2805-8. [Crossref] [PubMed]
- Warnock DG. Liddle syndrome: an autosomal dominant form of human hypertension. Kidney Int 1998;53:18-24. [Crossref] [PubMed]
- Firsov D, Schild L, Gautschi I, et al. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci U S A 1996;93:15370-5. [Crossref] [PubMed]
- Kellenberger S, Gautschi I, Rossier BC, et al. Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J Clin Invest 1998;101:2741-50. [Crossref] [PubMed]
- Snyder PM. Liddle's syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na(+) channel to the cell surface. J Clin Invest 2000;105:45-53. [Crossref] [PubMed]
- Anantharam A, Tian Y, Palmer LG. Open probability of the epithelial sodium channel is regulated by intracellular sodium. J Physiol 2006;574:333-47. [Crossref] [PubMed]
- Salih M, Gautschi I, van Bemmelen MX, et al. A Missense Mutation in the Extracellular Domain of αENaC Causes Liddle Syndrome. J Am Soc Nephrol 2017;28:3291-9. [Crossref] [PubMed]
- Enslow BT, Stockand JD, Berman JM. Liddle's syndrome mechanisms, diagnosis and management. Integr Blood Press Control 2019;12:13-22. [Crossref] [PubMed]
- Palmer BF, Alpern RJ. Liddle's syndrome. Am J Med 1998;104:301-9. [Crossref] [PubMed]
- Fan P, Zhang D, Pan XC, et al. Premature Stroke Secondary to Severe Hypertension Results from Liddle Syndrome Caused by a Novel SCNN1B Mutation. Kidney Blood Press Res 2020;45:603-11. [Crossref] [PubMed]
- O'Shaughnessy KM. Gordon Syndrome: a continuing story. Pediatr Nephrol 2015;30:1903-8. [Crossref] [PubMed]
- Gordon RD, Geddes RA, Pawsey CG, et al. Hypertension and severe hyperkalaemia associated with suppression of renin and aldosterone and completely reversed by dietary sodium restriction. Australas Ann Med 1970;19:287-94. [Crossref] [PubMed]
- Gordon RD, Hodsman GP. The syndrome of hypertension and hyperkalaemia without renal failure: long term correction by thiazide diuretic. Scott Med J 1986;31:43-4. [Crossref] [PubMed]
- Kahle KT, Wilson FH, Leng Q, et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 2003;35:372-6. [Crossref] [PubMed]
- Choate KA, Kahle KT, Wilson FH, et al. WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl- -transporting epithelia. Proc Natl Acad Sci U S A 2003;100:663-8. [Crossref] [PubMed]
- Wade JB, Fang L, Liu J, et al. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci U S A 2006;103:8558-63. [Crossref] [PubMed]
- Osawa M, Ogura Y, Isobe K, et al. CUL3 gene analysis enables early intervention for pediatric pseudohypoaldosteronism type II in infancy. Pediatr Nephrol 2013;28:1881-4. [Crossref] [PubMed]
- Gordon RD. Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension 1986;8:93-102. [Crossref] [PubMed]
- Mayan H, Munter G, Shaharabany M, et al. Hypercalciuria in familial hyperkalemia and hypertension accompanies hyperkalemia and precedes hypertension: description of a large family with the Q565E WNK4 mutation. J Clin Endocrinol Metab 2004;89:4025-30. [Crossref] [PubMed]
- Gamba G. Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. Am J Physiol Renal Physiol 2005;288:F245-52. [Crossref] [PubMed]
- Wilson RC, Krozowski ZS, Li K, et al. A mutation in the HSD11B2 gene in a family with apparent mineralocorticoid excess. J Clin Endocrinol Metab 1995;80:2263-6. [PubMed]
- Werder E, Zachmann M, Vollmin J, et al. Unusual steroid excretion in a child with low renin hypertension. Res Steroids 1974;6:385-9.
- Wilson RC, Dave-Sharma S, Wei JQ, et al. A genetic defect resulting in mild low-renin hypertension. Proc Natl Acad Sci U S A 1998;95:10200-5. [Crossref] [PubMed]
- Dave-Sharma S, Wilson RC, Harbison MD, et al. Examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J Clin Endocrinol Metab 1998;83:2244-54. [Crossref] [PubMed]
- New MI, Wilson RC. Steroid disorders in children: congenital adrenal hyperplasia and apparent mineralocorticoid excess. Proc Natl Acad Sci U S A 1999;96:12790-7. [Crossref] [PubMed]
- Li A, Tedde R, Krozowski ZS, et al. Molecular basis for hypertension in the "type II variant" of apparent mineralocorticoid excess. Am J Hum Genet 1998;63:370-9. [Crossref] [PubMed]
- Morineau G, Sulmont V, Salomon R, et al. Apparent mineralocorticoid excess: report of six new cases and extensive personal experience. J Am Soc Nephrol 2006;17:3176-84. [Crossref] [PubMed]
- Nunez BS, Rogerson FM, Mune T, et al. Mutants of 11beta-hydroxysteroid dehydrogenase (11-HSD2) with partial activity: improved correlations between genotype and biochemical phenotype in apparent mineralocorticoid excess. Hypertension 1999;34:638-42. [Crossref] [PubMed]
- White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 1997;18:135-56. [PubMed]
- Funder JW. Apparent mineralocorticoid excess. J Steroid Biochem Mol Biol 2017;165:151-3. [Crossref] [PubMed]
- Geller DS, Farhi A, Pinkerton N, et al. Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science 2000;289:119-23. [Crossref] [PubMed]
- Bollag WB. Regulation of aldosterone synthesis and secretion. Compr Physiol 2014;4:1017-55. [Crossref] [PubMed]
- Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101:1889-916. [Crossref] [PubMed]
- Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med 2004;351:33-41. [Crossref] [PubMed]
- Zennaro MC, Boulkroun S, Fernandes-Rosa FL. Pathogenesis and treatment of primary aldosteronism. Nat Rev Endocrinol 2020;16:578-89. [Crossref] [PubMed]
- Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2018;6:41-50. [Crossref] [PubMed]
- Savard S, Amar L, Plouin PF, et al. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension 2013;62:331-6. [Crossref] [PubMed]
- Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005;45:1243-8. [Crossref] [PubMed]
- Rossi GP, Sechi LA, Giacchetti G, et al. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab 2008;19:88-90. [Crossref] [PubMed]
- Born-Frontsberg E, Reincke M, Rump LC, et al. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn's Registry. J Clin Endocrinol Metab 2009;94:1125-30. [Crossref] [PubMed]
- Monticone S, Burrello J, Tizzani D, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol 2017;69:1811-20. [Crossref] [PubMed]
- Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient's cohorts and in population-based studies--a review of the current literature. Horm Metab Res 2012;44:157-62. [Crossref] [PubMed]
- Mosso L, Carvajal C, González A, et al. Primary aldosteronism and hypertensive disease. Hypertension 2003;42:161-5. [Crossref] [PubMed]
- Mulatero P, Tizzani D, Viola A, et al. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (Primary Aldosteronism in TOrino-GENetic forms). Hypertension 2011;58:797-803. [Crossref] [PubMed]
- Genovesi S, Antolini L, Orlando A, et al. Aldosterone-to-renin ratio depends on age and sex in children attending a clinic for cardiovascular risk assessment. J Hypertens 2018;36:344-52. [Crossref] [PubMed]
- Mattsson C, Young WF Jr. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol 2006;2:198-230. [Crossref] [PubMed]
- Sutherland DJ, Ruse JL, Laidlaw JC. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can Med Assoc J 1966;95:1109-19. [PubMed]
- Hirsch JS, Hong S. The Demystification of Secondary Hypertension: Diagnostic Strategies and Treatment Algorithms. Curr Treat Options Cardiovasc Med 2019;21:90. [Crossref] [PubMed]
- Lifton RP, Dluhy RG, Powers M, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 1992;355:262-5. [Crossref] [PubMed]
- Lifton RP, Dluhy RG, Powers M, et al. Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet 1992;2:66-74. [Crossref] [PubMed]
- Pascoe L, Curnow KM, Slutsker L, et al. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci U S A 1992;89:8327-31. [Crossref] [PubMed]
- Dluhy RG, Anderson B, Harlin B, et al. Glucocorticoid-remediable aldosteronism is associated with severe hypertension in early childhood. J Pediatr 2001;138:715-20. [Crossref] [PubMed]
- Litchfield WR, Anderson BF, Weiss RJ, et al. Intracranial aneurysm and hemorrhagic stroke in glucocorticoid-remediable aldosteronism. Hypertension 1998;31:445-50. [Crossref] [PubMed]
- Shahrrava A, Moinuddin S, Boddu P, et al. A Case of Glucocorticoid Remediable Aldosteronism and Thoracoabdominal Aneurysms. Case Rep Endocrinol 2016;2016:2017571. [Crossref] [PubMed]
- Al Romhain B, Young AM, Battacharya JJ, et al. Intracranial aneurysm in a patient with glucocorticoid-remediable aldosteronism. Br J Neurosurg 2015;29:715-7. [Crossref] [PubMed]
- Gates LJ, Benjamin N, Haites NE, et al. Is random screening of value in detecting glucocorticoid-remediable aldosteronism within a hypertensive population? J Hum Hypertens 2001;15:173-6. [Crossref] [PubMed]
- Dluhy RG, Lifton RP. Glucocorticoid-remediable aldosteronism (GRA): diagnosis, variability of phenotype and regulation of potassium homeostasis. Steroids 1995;60:48-51. [Crossref] [PubMed]
- Mulatero P, di Cella SM, Williams TA, et al. Glucocorticoid remediable aldosteronism: low morbidity and mortality in a four-generation italian pedigree. J Clin Endocrinol Metab 2002;87:3187-91. [Crossref] [PubMed]
- Stowasser M, Bachmann AW, Huggard PR, et al. Treatment of familial hyperaldosteronism type I: only partial suppression of adrenocorticotropin required to correct hypertension. J Clin Endocrinol Metab 2000;85:3313-8. [Crossref] [PubMed]
- Stowasser M, Gordon RD, Tunny TJ, et al. Familial hyperaldosteronism type II: five families with a new variety of primary aldosteronism. Clin Exp Pharmacol Physiol 1992;19:319-22. [Crossref] [PubMed]
- Lafferty AR, Torpy DJ, Stowasser M, et al. A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22). J Med Genet 2000;37:831-5. [Crossref] [PubMed]
- Stowasser M, Wolley M, Wu A, et al. Pathogenesis of Familial Hyperaldosteronism Type II: New Concepts Involving Anion Channels. Curr Hypertens Rep 2019;21:31. [Crossref] [PubMed]
- Scholl UI, Stölting G, Schewe J, et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet 2018;50:349-54. [Crossref] [PubMed]
- Schewe J, Seidel E, Forslund S, et al. Elevated aldosterone and blood pressure in a mouse model of familial hyperaldosteronism with ClC-2 mutation. Nat Commun 2019;10:5155. [Crossref] [PubMed]
- Stowasser M, Gordon RD. Primary aldosteronism--careful investigation is essential and rewarding. Mol Cell Endocrinol 2004;217:33-9. [Crossref] [PubMed]
- Torpy DJ, Gordon RD, Lin JP, et al. Familial hyperaldosteronism type II: description of a large kindred and exclusion of the aldosterone synthase (CYP11B2) gene. J Clin Endocrinol Metab 1998;83:3214-8. [Crossref] [PubMed]
- New MI, Geller DS, Fallo F, et al. Monogenic low renin hypertension. Trends Endocrinol Metab 2005;16:92-7. [Crossref] [PubMed]
- Pons Fernández N, Moreno F, Morata J, et al. Familial hyperaldosteronism type III a novel case and review of literature. Rev Endocr Metab Disord 2019;20:27-36. [Crossref] [PubMed]
- Mulatero P, Tauber P, Zennaro MC, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension 2012;59:235-40. [Crossref] [PubMed]
- Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011;331:768-72. [Crossref] [PubMed]
- Geller DS, Zhang J, Wisgerhof MV, et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 2008;93:3117-23. [Crossref] [PubMed]
- Funder JW. Primary Aldosteronism. Hypertension 2019;74:458-66. [Crossref] [PubMed]
- Monticone S, Hattangady NG, Nishimoto K, et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab 2012;97:E1567-72. [Crossref] [PubMed]
- Mashmoushi A, Choudhary A, Thomas CP, et al. A rare case of hyporeninemic hypertension: Questions. Pediatr Nephrol 2021;36:567-8. [Crossref] [PubMed]
- Mashmoushi A, Choudhary A, Thomas CP, et al. A rare case of hyporeninemic hypertension: Answers. Pediatr Nephrol 2021;36:569-73. [Crossref] [PubMed]
- Charmandari E, Sertedaki A, Kino T, et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab 2012;97:E1532-9. [Crossref] [PubMed]
- Monticone S, Bandulik S, Stindl J, et al. A case of severe hyperaldosteronism caused by a de novo mutation affecting a critical salt bridge Kir3.4 residue. J Clin Endocrinol Metab 2015;100:E114-8. [Crossref] [PubMed]
- Scholl UI, Stölting G, Nelson-Williams C, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife 2015;4:e06315. [Crossref] [PubMed]
- Daniil G, Fernandes-Rosa FL, Chemin J, et al. CACNA1H Mutations Are Associated With Different Forms of Primary Aldosteronism. EBioMedicine 2016;13:225-36. [Crossref] [PubMed]
- Boulkroun S, Fernandes-Rosa FL, Zennaro MC. Old and new genes in primary aldosteronism. Best Pract Res Clin Endocrinol Metab 2020;34:101375. [Crossref] [PubMed]
- Scholl UI, Goh G, Stölting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 2013;45:1050-4. [Crossref] [PubMed]
- Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 2013;45:1055-60. [Crossref] [PubMed]
- Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 2013;45:440-4, 444.e1-2.
- Rhayem Y, Perez-Rivas LG, Dietz A, et al. PRKACA Somatic Mutations Are Rare Findings in Aldosterone-Producing Adenomas. J Clin Endocrinol Metab 2016;101:3010-7. [Crossref] [PubMed]
- Peterson RE, Imperato-McGinley J, Gautier T, et al. Male pseudohermaphroditism due to multiple defects in steroid-biosynthetic microsomal mixed-function oxidases. A new variant of congenital adrenal hyperplasia. N Engl J Med 1985;313:1182-91. [Crossref] [PubMed]
- Chua SC, Szabo P, Vitek A, et al. Cloning of cDNA encoding steroid 11 beta-hydroxylase (P450c11). Proc Natl Acad Sci U S A 1987;84:7193-7. [Crossref] [PubMed]
- Hurley DM, Accili D, Stratakis CA, et al. Point mutation causing a single amino acid substitution in the hormone binding domain of the glucocorticoid receptor in familial glucocorticoid resistance. J Clin Invest 1991;87:680-6. [Crossref] [PubMed]
- Charmandari E, Kino T, Souvatzoglou E, et al. Natural glucocorticoid receptor mutants causing generalized glucocorticoid resistance: molecular genotype, genetic transmission, and clinical phenotype. J Clin Endocrinol Metab 2004;89:1939-49. [Crossref] [PubMed]
Cite this article as: Mashmoushi A, Wolf MTF. A narrative review of Hyporeninemic hypertension—an indicator for monogenic forms of hypertension. Pediatr Med 2022;5:21.

