
Validity of anti-reflux formulas as a slightly thick liquid: effect of time, caloric density, and refrigerated storage on formula thickness
Introduction
Advancements in neonatal medicine have resulted in an increased prevalence of infants suffering from swallowing deficits (dysphagia) (1-3). These deficits, if left untreated, can cause a myriad of harmful health effects including aspiration, cardiopulmonary instability, and lengthened hospital stays (4-8). One of the most effective ways of treating these deficits is to thicken the infant’s formula (4,9,10). Commonly used thickeners for infants include infant cereals, such as rice or oatmeal cereal, or commercial thickeners, such as those based in xanthan gum, carob bean, or corn starch (11). Unfortunately, many of the thickening agents used to create thickened liquids have been postulated to carry negative health effects such as the risk of arsenic exposure, necrotizing enterocolitis, reduced nutrient absorption, and changes to bowel movements (11-15). Concern for these harmful side effects often prevents their use among the young neonates who often need them the most such as those who are less than 42 weeks postmenstrual age or those with a history of necrotizing enterocolitis (11).
One method to provide infants with the benefit of thickened liquids without the clinician’s addition of these prohibited agents is through the use of anti-reflux formulas (10,16). These FDA approved formulas are manufactured with incorporated thickening agents for management of gastroesophageal reflux. Research examining the validity of these formulas for the treatment of dysphagia indicates 20 kcal/oz Ready to Feed Enfamil A.R.™, a commonly used formula in the hospital setting, qualifies as a thickened liquid based on international thickened liquid standards (10,16). While these findings are promising, their clinical applicability is limited by the failure to test thickness under common clinical conditions known to impact liquid thickness such as formula refrigeration, caloric density, and time after mixing (2,17). Elucidating the effects of these common clinical conditions is critical in ensuring infants are receiving the desired dysphagia treatment, as providing a liquid that is not thick enough can cause deleterious health effects if aspirated, and providing a liquid that is too thick can impede an infant’s ability to express the formula from the bottle nipple to meet nutritional needs.
The objective of the current investigation was to test the effect of these common clinical variables on thickness of two commonly used U.S. anti-reflux formulas (Enfamil A.R.™ and Similac Spit-Up®) in their ready to feed and powder formulations. To do this we completed an investigation guided by the following aims: (I) test the effect of time on thickness of anti-reflux formula; (II) test the effect of caloric density on thickness of anti-reflux formula; and (III) test the effect of refrigerated storage on anti-reflux formula thickness. To maintain clinical relevancy, liquid thickness was quantified using the International Dysphagia Diet Initiative testing method and nomenclature (18). We present the following article in accordance with the Hybrid STROBE & STROBE-ME checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-44/rc).
Methods
Two commonly used anti-reflux infant formulas (Enfamil A.R.™ and Similac Spit-Up®) were used in this investigation. Corresponding non-reflux formula correlates were also tested (Enfamil Infant™ and Similac Advance®) to elucidate the effect of these variables under typical formula conditions. All formulas were tested in their ready to feed 20 kcal/oz variants, which are commonly provided in acute care environments, as well as their powder variants, which are commonly provided in home settings. Ready to feed variants were tested at room temperature in the research lab (68.2–72.7℉) and shaken prior to testing per manufacturer instructions. To test the effect of caloric density on formula thickness, powder formulas were mixed with graduated caloric densities ranging from 20–30 kcal/oz. Manufacturer instructions provided recipes for 20 kcal/oz variants. Recipes for higher calorie counts ranging from 22–30 kcal/oz were provided by Boston Children’s Hospital Center for Nutrition. See Table 1 for a listing of formulations. Of note, although anti-reflux formulations >24 kcal/oz were tested in the current investigation for scientific inquiry, manufacturer guidelines indicate these should not be provided to infants clinically due to the resulting formula thickness. All powder formulas were mixed according to manufacturer instructions. Specifically, an unpacked level scoop of powder was added to the designated amount of room temperature water (64–72 ℉) that had been measured by a Volu-feed® to ensure accuracy. Formula was shaken immediately after combination until all powder was visibly dissolved. Enfamil™ formulas were shaken 5 minutes after mixing and Similac formulas were swirled 2 minutes after mixing per manufacturer instructions.
Table 1
| Caloric density (kcal/oz) | Water (oz) | Formula (scoops) |
|---|---|---|
| Enfamil Infant™ | ||
| 20 | 2 oz | 1 |
| 22 | 5 ½ oz | 3 |
| 24 | 6 ½ oz | 4 |
| 26 | 6 oz | 4 |
| 30 | 5 oz | 4 |
| Enfamil A.R.™ | ||
| 20 | 2 oz | 1 |
| 22 | 5 ½ oz | 3 |
| 24 | 5 oz | 3 |
| 26 | 6 oz | 4 |
| 28 | 5 ½ oz | 4 |
| 30 | 5 oz | 4 |
| Similac Advance® | ||
| 20 | 2 oz | 1 |
| 22 | 3 ½ oz | 2 |
| 24 | 5 oz | 3 |
| 27 | 5 ½ oz | 4 |
| 30 | 2 ½ oz | 2 |
| Similac Spit-Up® | ||
| 20 | 2 oz | 1 |
| 22 | 5 oz | 3 |
| 24 | 4 ½ oz | 3 |
| 26 | 5 ½ oz | 4 |
| 28 | 4 oz | 3 |
| 30 | 6 oz | 5 |
Of note, the recommendation of fortification and the determination of fortification recipes should strictly be administered at the discretion of a registered dietician and/or physician. Manufacturers do not recommend fortifying anti-reflux formulas above 24 kcal/oz.
Immediately following mixing, the formulas underwent gravity flow testing using International Dysphagia Diet Standardization Initiative (IDDSI) methodology (18). In contrast to viscosity testing, which quantifies a liquid’s viscosity using refined instrumentation not accessible in the clinical settings, IDDSI methodology provides an easy and reliable clinical method of determining functional categories of liquid thickness based on the residual volume of fluid that remains in a 10 mL syringe following 10 seconds of unconstrained vertical flow. The residual volume correlates to an IDDSI classification of thin, slightly thick, mildly thick (previously termed nectar), or moderately thick (previously termed honey). A residual volume of 0–1 mL is classified as thin, 1–4 mL as slightly thick, 4–8 mL as mildly thick, 8–10 mL as moderately thick. Syringes used in this investigation were IDDSI approved BD 10mL Luer-LokTM Tip syringe (REF 302995). Previous research indicates these IDDSI classification correspond to unique viscosity levels when tested using rheologic testing methods under graduated shear rates (50 s, thin =0.0867 Pa s, slightly thick =0.22 Pa s, mildly thick =0.404 Pa s) (19).Three gravity flow testing trials were completed for each testing condition according to IDDSI recommendations. To maintain clinical relevance where slight variations in mixing occur for each bottle that is created, a new batch of formula was mixed for each trial. Likewise, as previous research suggests liquids can change viscosity over time (2,17), the effect of time on formula thickness was studied by testing liquid thickness at 5-minute intervals over 30 minutes to approximate the maximum duration of a bottle feed. In doing so, each caloric density underwent 21 IDDSI tests (i.e., 20 kcal Enfamil AR was mixed and tested 3 different times, during which measures were taken at 0, 5, 10, 15, 20, 25, and 30 minutes). To test the effect of refrigerated storage, powdered formulas that are often mixed in large batches and refrigerated until use were refrigerated for 3 hours (40.3–45.2 ℉) and then tested after being warmed to room temperature (64–81 ℉) to simulate the time between when formula may be prepared by a caregiver and given to an infant. Warming was completed using the EivotorTM bottle warmer. Warmed formulas underwent additional serial gravity flow testing at 5-minute intervals for 30 minutes. Ready-to-feed formulations did not undergo testing in the refrigerated and warmed testing condition as clinical practice does not necessitate their refrigeration due to the presence of preservatives. Residual volume results were measured to the nearest. 2 mL based available syringe metrics, with results reported in the IDDSI category as well as the mean (minimum-maximum) residual volumes.
Statistical analysis
In addition to reporting liquid thickness in terms of IDDSI category, statistical analysis was completed to compare statistical differences in residual volumes. To test the effect of time and caloric density on the thickness of anti-reflux formula, we conducted a repeated-measures ANOVA on the three trials for each brand in the 20 kcal/oz formulations, and the three trials of each brand at each graduated caloric density. In these tests, the residual volume in mL served as the dependent variable, the 5-minute time intervals were within-subject factors, and brand and caloric density were between-subject factors. The effect of refrigeration on formula thickness was tested using a standard two-way ANOVA as only a single time point was used for the dependent variable. In each model, marginal means were estimated and pairwise comparisons were made with Bonferonni corrections for multiple comparisons.
Results
Non-Reflux Formulas: Enfamil Infant™ and Similac Advance®
Both non-reflux formulas were characterized as thin liquids throughout the entire 30-minute testing period in their 20 kcal/oz ready to feed and powder formulations. Increasing caloric density of powder formulas did not change liquid thickness, nor did refrigerated storage and warming (Enfamil™ 0 mL, Similac® 0 mL).
Anti-Reflux Formulas: Enfamil A.R.™ and Similac Spit-Up®
Time
Examination of anti-reflux formulas in their 20 kcal/oz ready to feed formulations revealed differences in thickness across brands, with Enfamil A.R.™ categorized as slightly thick (1.3, 1.1–1.5 mL) and Similac Spit-Up® categorized as thin (0.7, 0.4–0.8 mL) throughout the 30-minute testing period. Comparison of these values to those obtained from the powder correlates also revealed differences in thickness properties. Whereas Enfamil A.R.™ ready to feed remained slightly thick throughout the 30-minute testing period, its powder formulation started out as a thin liquid (0, 0–0 mL) followed by a steady increase in thickness over 30 minutes that resulted in a slightly thick liquid designation by 20 minutes (1.1, 0.7–1.4 mL). Similac Spit-Up® powder performed similarly to its ready to feed correlate and remained a thin liquid throughout the 30-minute testing period, however its residual volume was notably less in the powder formulation (0.0, 0.0–0.1 mL) than ready to feed (0.7, 0.4–0.8 mL). Figure 1 depicts the thickness of Enfamil A.R. ™ and Similac Spit Up® formula in their 20 kcal/oz ready to feed and powder formulations. Results from the repeated-measures ANOVA supported these findings, indicating significant differences in residual volume between Enfamil and Similac anti-reflux formula brands (P<0.001). Examination of how anti-reflux formula thickness changed with time revealed a significant thickening effect (P<0.001) that varied based on formula type. Specifically, time after mixing had a large impact on Enfamil A. R. Powder thickness (P<0.01), whereas there was no significant effect of time in Enfamil A. R. ready-to-feed formulation, or either formulations of Similac Spit-Up (P=0.255).
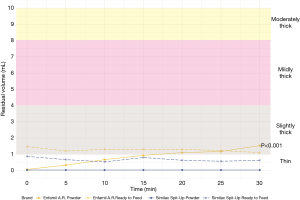
Caloric density
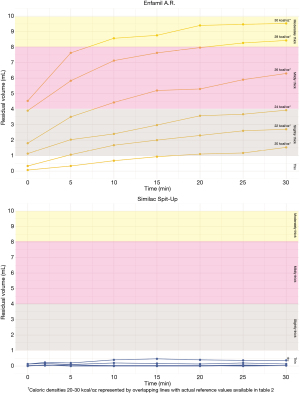
Increasing caloric density of the powder formulas had different effects across anti-reflux formula brands (P<0.001). Table 2 provides IDDSI values of formulas at each caloric density. Enfamil A.R.™ exhibited a stepwise increase in thickness with every 2 kcal/oz increase of caloric density beyond 22 kcal/oz (P<0.001). All formulations of Enfamil A.R. increased in thickness throughout the 30-minute testing period, which resulted in a change in IDDSI thickness level between the first five minutes and 30 minutes following mixing. In contrast, Similac Spit-Up® did not exhibit any change in thickness based on caloric density (P=0.389). Figure 2 depicts Enfamil A.R.™ and Similac Spit-Up® thickness levels at each caloric density throughout the 30-minute testing period.
Table 2
| Caloric density (kcal/oz) | Initial thickness | Ending thickness |
|---|---|---|
| Enfamil A.R. ™ | ||
| 20 kcal/oz | Thin (0.1, 0.0–0.1) | Slightly Thick (1.5, 1.2–1.8) |
| 22 kcal/oz | Thin (0.3, 0.0–0.6) | Slightly Thick (2.7, 1.4–3.6) |
| 24 kcal/oz | Slightly Thick (1.1, 1.0–1.4) | Mildly Thick (3.9, 3.0–4.8) |
| 26 kcal/oz | Slightly Thick (1.8, 1.6–2.2) | Mildly Thick (6.3, 5.5–7.2) |
| 28 kcal/oz | Slightly Thick (3.9, 3.2–4.3) | Moderately Thick (8.4, 7.7–9.0) |
| 30 kcal/oz | Mildly Thick (4.53, 4.3–5.0) | Moderately Thick (9.5, 9.2–9.8) |
| Similac Spit-Up® | ||
| 20 | Thin (0.0, 0.0–0.1) | Thin (0.0, 0.0–0.1) |
| 22 | Thin (0.0, 0.0–0.1) | Thin (0.0, 0.0–0.1) |
| 24 | Thin (0, 0.0–0.0) | Thin (0.0, 0.0–0.1) |
| 26 | Thin (0.0, 0.0–0.1) | Thin (0.0, 0.0–0.1) |
| 28 | Thin (0.1, 0.1–0.2) | Thin (0.0, 0.0–0.2) |
| 30 | Thin (0.1, 0.1–0.2) | Thin (0.4, 0.3–0.4) |
Values indicate average (min-max) residual volume in milliliters immediately after mixing (initial thickness) and at the end of the 30-minute testing period (ending thickness).
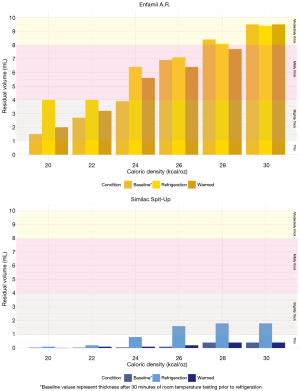
Refrigerated storage
Both Enfamil A.R.™ and Similac Spit-Up® showed non-significant trends of increased thickness when tested cold after three hours of refrigeration, followed by thinning after being re-heated to ‘baseline’ pre-refrigeration values measured 30 minutes after mixing (P=0.259) (Figure 3). The magnitude of change in thickness between baseline and cold post-refrigeration conditions was influenced by formula type and caloric density. Specifically, Enfamil A.R.™ exhibited a greater magnitude of change between baseline and cold post refrigeration condition (0.9 mL) than did Similac Spit-Up® (0.7 mL).
Discussion
In this investigation we explored how common clinical conditions impact the thickness of two anti-reflux formulas frequently used in the United States. Our findings indicate (I) Similac Spit-Up® is not an effective thickening option in either ready to feed or powdered formulations; (II) Ready to feed Enfamil A.R.™ is a valid slightly thick formula option that remains consistent in thickness throughout the duration of a bottle feed; (III) The thickness of powdered Enfamil A.R.™ is dependent on caloric density and time since mixing; and (IV) The thickness of powdered Enfamil A.R.™ is retained following refrigerated storage for three hours and warming to room temperature.
Previous investigations examining the thickness of these anti-reflux formulas in their 20 kcal/oz formulations have yielded similar results. Rheological testing of powder formulas by Frazier et al. (2016) indicated that while the non-reflux formula Enfamil Infant™ qualified as a thin liquid according to National Dysphagia Diet criterion, its anti-reflux counterpart Enfamil A.R.™ had a much higher viscosity that placed it between the thin and nectar thick (IDDSI mildly thick) liquid range (10). More recent work by Pados et al. (2021) using the IDDSI flow testing and classification approach also found the non-reflux formulas Enfamil Infant™ and Similac Advance to qualify as thin liquids in both 20 kcal/oz powder and ready to feed formulations (16). However, in contrast to Frazier et al. (2016), they found Enfamil A.R.™ in its ready to feed formulation was the only anti-reflux formula to meet IDDSI thickened liquid criterion, with its powder formulation classifying as thin (16). In the current investigation we found that both powder and ready to feed formulations of Enfamil A.R.™ reached IDDSI thickened liquid criteria, though they differed in their time to reach this designation. Specifically, the ready to feed formulation classified as a slightly thick liquid immediately, whereas the powdered formulation did not reach this classification until 20 minutes after mixing. Based on these findings, it is plausible that these discrepancies may be a result of methodological differences pertaining to when thickness was tested across investigations.
The clinical implications of the aforementioned discrepancies between powder and ready to feed formulas thickness levels are potentially quite significant. It is common practice for neonates to be fed using ready to feed formulas while in the acute care hospital setting due to ease of administration, and to then transition to the powdered formulation upon discharge into the home setting. Historically this transition in formulations was thought to only pose an impact to the caregiver who needs to mix the powdered formula prior to administration. Our results indicate this transition holds potentially deleterious implications for the infant as well, as the powdered formulation of Enfamil A.R.™ does not become a slightly thick liquid until 20 minutes after mixing. Based on these findings, caregivers of infants who are being provided Enfamil A.R.™ 20 kcal/oz as a dysphagia treatment should be instructed to wait 20 minutes prior to powder formula administration to allow sufficient thickening time, or if the medical team deems appropriate, providing a higher caloric density variant that will provide the necessary thickness immediately.
The changes in powder Enfamil A.R.™ thickness level over time also has implications for milk expression. Our findings indicate that although ready to feed Enfamil A.R.™ remains slightly thick throughout the duration of a typical bottle feed, the powder formulation continues to thicken. This discrepancy between formulations may be a result of the addition of emulsifiers and preservatives such as Carrageenan (found in ready to feed formulations) and Mono/diglycerides, or the result of heat sterilization processes that the ready to feed formulations undergo (20). Regardless of the source, a formula that increases in thickness throughout a feed has the potential to impede an infant’s ability to express the liquid as the feed progresses. This is a critical concept to consider, as maintenance of airway protection is important, however if it comes at the demise of an infant’s ability to meet full nutritional needs it is not a functional intervention. Likewise, it has been postulated that too thick of liquids impose a greater gastric burden and could have unintended harmful implications. Future investigations are necessary to examine how these slight changes in thickness impact milk flow rate and milk ingestion to determine the viability of this home treatment option.
Time after mixing, however, was not the only clinical variable that was found to alter thickness of powder Enfamil A.R.™. Fortification was found to also have a stepwise effect, increasing its thickness with every 2 unit increase in caloric density. The magnitude of this change was quite robust, with formulations at 20 kcal/oz classifying as slightly thick at the end of the 30-minute testing period, whereas those prepared at 30 kcal/oz classifying as moderately thick at the end of the 30-minute testing period. Fortification of infant formulas to higher caloric densities is a common practice among preterm infants to enable appropriate nutrient profiles to be obtained. As such, having a reference for the subsequent changes this practice will have on formula thickness level is critical for selecting a bottle nipple that will enable the infant to express the milk efficiently. Future interdisciplinary research collaborations with experts in nutrition are critical in determining if fortification of Enfamil A.R.™ has the potential to provide a safe thickening option for those infants who require liquids greater than slightly thick. Although this investigation tested anti-reflux formulations fortified up to 30 kcal/oz out of scientific inquiry, it is critical to note that fortification of anti-reflux formulas to caloric densities greater than 24 kcal/oz is not recommended by formula manufacturers, and therefore such practice should not be implemented unless guided by a physician and nutrition team.
This investigation was limited by several variables, the greatest of which is the lack of data pertaining to the effects of warming on anti-reflux formula thickness. Although warming of formula was used after refrigeration storage, this was done solely to return the formula to a room temperature that would not preclude an infant from drinking it. Research indicates liquids become thinner with warming, and therefore it is likely the results found in this investigation may differ under these testing conditions. Future work examining how these clinical variables impact thickness level of warmed liquids is critical in guiding clinical practice among infants who are administered formula this way. Another limitation of our investigation pertains to the conclusions that can be drawn regarding the effect of refrigerated storage on formula thickness. While we found trends of increased formula thickness after a three-hour refrigeration period, our failure to include a control arm of formula that was room temperature for 3 hours limits our ability to determine if the three hours of time is what increased formula thickness, or if it is the refrigeration process. This approach was not pursued in this investigation as the formula is intended to be refrigerated after preparation, and therefore completing this testing would strictly have scientific significance but none clinically at this time. Lastly, a key area that requires further investigation is the clinical significance of slight changes in liquid thickness on swallow physiology and function. Videofluoroscopic exams determine infant safety for a given liquid classes used barium contrast prepared at standardized thin, nectar, and honey viscosities. As demonstrated in this investigation, thickness of liquids under clinical conditions is highly variable, and often is slightly thicker or thinner than those conditions tested under fluoroscopy. Determining the significance of these slight differences is critical in determining how much variability is acceptable and the rigor that testing needs to be completed prior to each feed.
Conclusions
In this investigation we demonstrated common clinical variables including formula brand, caloric density, powder vs. ready to feed formulation, time after mixing can greatly impact the thickness of anti-reflux infant formula. Failure to consider these variables has the potential to greatly impede achievement of the desired treatment effect, as insufficient thickening may result in aspiration, and over thickening may impede an infant’s ability to meet nutritional needs. Future investigations examining the safety and efficacy of using anti-reflux formulas for the treatment of neonatal swallowing problems is a critical next step in establishing evidence-based treatment regimens for these fragile populations.
Acknowledgments
This work was supported by the work of Anna Maunu, who assisted in data collection, as well as Jane Riebold and Catharine Seiler who provided the recipes for preparing formula at higher caloric density concentrations.
Funding: This work was supported by the University of Minnesota Undergraduate Research Opportunities Program (UROP).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Steven M. Barlow) for the series “Neonatal Feeding and Developmental Issues” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Hybrid STROBE & STROBE-ME reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-44/rc
Data Sharing Statement: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-44/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-44/coif). The series “Neonatal Feeding and Developmental Issues” was commissioned by the editorial office without any funding or sponsorship. KEM is employed by University of Minnesota and has received speakers’ fees for presenting this work at National Association of Neonatal Therapists conference. Her lab received a stipend through the University of Minnesota Undergraduate Research Opportunities Program (UROP) for the execution of this work. AS and AS received a stipend through the University of Minnesota Undergraduate Research Opportunities Program to complete this work, and also received a travel award from Society for Ear Nose and Throat Advances in Children to present this work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Behrman R, Butler A. Institute of Medicine (US) Committee on Understanding Premature Birth: Causes, Conseqeunces, and Prevention. 2007.
- Gosa MM, Dodrill P. Effect of Time and Temperature on Thickened Infant Formula. Nutr Clin Pract 2017;32:238-44. [Crossref] [PubMed]
- Horton J, Atwood C, Gnagi S, et al. Temporal Trends of Pediatric Dysphagia in Hospitalized Patients. Dysphagia 2018;33:655-61. [Crossref] [PubMed]
- Duncan DR, Larson K, Davidson K, et al. Feeding Interventions Are Associated With Improved Outcomes in Children With Laryngeal Penetration. J Pediatr Gastroenterol Nutr 2019;68:218-24. [Crossref] [PubMed]
- Dodrill P, Gosa MM. Pediatric Dysphagia: Physiology, Assessment, and Management. Ann Nutr Metab 2015;66:24-31. [Crossref] [PubMed]
- Poets CF, Langner MU, Bohnhorst B. Effects of bottle feeding and two different methods of gavage feeding on oxygenation and breathing patterns in preterm infants. Acta Paediatr 1997;86:419-23. [Crossref] [PubMed]
- Radford PJ, Stillwell PC, Blue B, et al. Aspiration complicating bronchopulmonary dysplasia. Chest 1995;107:185-8. [Crossref] [PubMed]
- Han C, Shin J, Jeon GW. Development of Swallowing Function in Infants with Oral Feeding Difficulties. Int J Pediatr 2020;2020:5437376. [Crossref] [PubMed]
- McGrattan KE, McGhee H, DeToma A, et al. Dysphagia in infants with single ventricle anatomy following stage 1 palliation: Physiologic correlates and response to treatment. Congenit Heart Dis 2017;12:382-8. [Crossref] [PubMed]
- Frazier J, Chestnut AH, Jackson A, et al. Understanding the Viscosity of Liquids used in Infant Dysphagia Management. Dysphagia 2016;31:672-9. [Crossref] [PubMed]
- Duncan DR, Larson K, Rosen RL. Clinical Aspects of Thickeners for Pediatric Gastroesophageal Reflux and Oropharyngeal Dysphagia. Curr Gastroenterol Rep 2019;21:30. [Crossref] [PubMed]
- Salvatore S, Savino F, Singendonk M, et al. Thickened infant formula: What to know. Nutrition 2018;49:51-6. [Crossref] [PubMed]
- Krummrich P, Kline B, Krival K, et al. Parent perception of the impact of using thickened fluids in children with dysphagia. Pediatr Pulmonol 2017;52:1486-94. [Crossref] [PubMed]
- González-Bermúdez CA, Frontela-Saseta C, López-Nicolás R, et al. Effect of adding different thickening agents on the viscosity properties and in vitro mineral availability of infant formula. Food Chem 2014;159:5-11. [Crossref] [PubMed]
- Bosscher D, Van Caillie-Bertrand M, Deelstra H. Do thickening properties of locust bean gum affect the amount of calcium, iron and zinc available for absorption from infant formula? In vitro studies. Int J Food Sci Nutr 2003;54:261-8. [Crossref] [PubMed]
- Pados BF, Feaster V. Effect of Formula Type and Preparation on International Dysphagia Diet Standardisation Initiative Thickness Level and Milk Flow Rates From Bottle Teats. Am J Speech Lang Pathol 2021;30:260-5. [Crossref] [PubMed]
- Garcia JM, Chambers E 4th, Matta Z, et al. Viscosity measurements of nectar- and honey-thick liquids: product, liquid, and time comparisons. Dysphagia 2005;20:325-35. [Crossref] [PubMed]
- Cichero JA, Lam P, Steele CM, et al. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia 2017;32:293-314. [Crossref] [PubMed]
- Hanson B, Jamshidi R, Redfearn A, et al. Experimental and Computational Investigation of the IDDSI Flow Test of Liquids Used in Dysphagia Management. Ann Biomed Eng 2019;47:2296-307. [Crossref] [PubMed]
- Necas J, Bartosikova L. Carrageenan: a review. Veterinarni Medicina 2013;58:187-205. [Crossref]
Cite this article as: McGrattan KE, Spoden A, Sterkowitz A, Gosa MM, Beckstrand M, Hernandez K. Validity of anti-reflux formulas as a slightly thick liquid: effect of time, caloric density, and refrigerated storage on formula thickness. Pediatr Med 2022;5:14.

