
Photobiomodulation for pediatric hypertrophic tonsils: a clinical case report
Introduction
Photobiomodulation (PBM) has been used for the treatment of surgical wounds, nerve regeneration, chronic pain, and sports injuries with evidence of its efficacy continuing to accumulate (1-16). However, the use of PBM for the treatment of snoring and obstructive sleep apnea (OSA) is a relatively recent new development, with the first literature review on PBM and sleep breathing disorders published in 2020 (17). While there are case reports of PBM resulting in improvement in OSA patients, the research is both new and controversial (17-20). This is likely from current clinical data showing that PBM intervention does not necessarily reduce the Apnea-Hypopnea Index, commonly used in the diagnosis of OSA, for every patient and that it is not yet known which patients may benefit and/or what selection criteria should be used to predict which patients may respond favourably to PBM as a treatment for OSA. This is not surprising given the relative youth of this modality of treatment, and further research to more thoroughly investigate PBM for OSA is already underway.
OSA is not a disease limited to adults; pediatric obstructive sleep apnea (POSA) has different etiology from adult OSA (21-25). While impaired anatomy is essential for adult OSA, a majority of children who suffer from POSA present with hypertrophic tonsils (26-31). This difference in etiology explains why first line treatment for adult OSA is usually the use of positive airway pressure machines, while for POSA first line treatment is usually surgical removal of hypertrophic tonsils and adenoids (28,32-37).
Tonsil and adenoid (T&A) surgery, both partial removal (tonsillotomy) and complete removal (tonsillectomy) presents with its own risks, both short- and long-term. Short-term risks of T&A surgery include secondary hemorrhage, complications with surgical anesthesia, and tonsillar regrowth from partial removal (37-39). Long-term risks include the development of respiratory infections and infectious diseases (36). Therefore, any treatments that would reduce the risks of T&A surgery should be explored, including the possibility of using PBM to treat pediatric hypertrophic tonsils.
We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/pm-21-18).
Case presentation
A 12-year-old male presented for routine dental examination in February 2019. The patient’s mother reported the patient suffered from snoring, poor sleep, nocturnal bruxism, chronic mouth breathing, and persistent ear infections. Visual intraoral examination showed the patient with bilateral grade 3 tonsillar swelling. The patient’s mother was informed of the clinical findings and reported that the child was already referred and scheduled for an otolaryngology consult for evaluation and potential treatment of the child’s hypertrophic tonsils. Upon further discussion the child’s mother indicated that the child had not undergone any formal overnight sleep testing for sleep disordered breathing; despite the child scoring positive for sleep disorders on the BEARS Sleep Screening Tool and 9/22 on the PSQ (Figure 1) for a high likelihood for pediatric sleep apnea, no consultation with a pediatric sleep specialist or a pediatric sleep study had yet been performed or ordered (40,41). The child’s mother requested to know other treatment options, including whether the use of PBM methods for adult OSA and snoring would be effective for POSA. The child’s mother was informed no research into this area existed and that PBM for the child’s hypertrophic tonsils could be attempted, but that clinical evaluation by an appropriate physician was still necessary. The child’s mother requested and consented to the use of PBM for the child’s hypertrophic tonsils.
The child and child’s mother were provided information about the procedure and informed consent was obtained. The child was reclined supine in a dental chair, no sedation or topical anesthetic was necessary. PBM treatment with a Fotona LightWalker laser was provided using a fractional laser beam delivered with a R30 and PS04 handpiece at minimally invasive settings according to manufacturer’s instructions modified for pediatric delivery. The laser therapy was manually delivered transorally directly targeted at the hypertrophic tonsillar tissue at 10 Hz in LP mode. The laser was delivered in slow horizontal and vertical passes ensuring significant delivery overlap. Treatment took a total of ten minutes. The child reported feeling no ill effects to treatment. The child returned for re-evaluation three months after treatment, and also reported feeling no ill effects from treatment during the 3-month interval.
Clinical photos were taken immediately before, immediately after, at a three month follow-up appointment, and one year after the intervention. Pre-treatment visual grading showed tonsils to be grade 3 bilaterally (Figure 2A). Immediate post-treatment visual grading showed tonsils to be grade 1 bilaterally (Figure 2B). Follow-up visual grading at the three month interval showed tonsils to be grade 1/2 bilaterally (Figure 2C). Follow-up visual grading one year after intervention showed tonsils to be grade 1 bilaterally (Figure 2D).
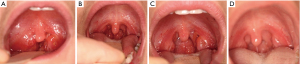
Immediately after treatment, the child reported “mom, I can breathe now”.
At the three month follow-up, the child’s mother reported that the child was sleeping better, less hyperactive, no longer mouth breathing, better able to concentrate, and that she could no longer hear the child bruxing during the night (Table 1). Follow-up screening on the BEARS Sleep Screening Tool was negative and scoring on the PSQ was 1/21, with 1 non-response (Figure 1).
Table 1
| Mother reported symptoms pre-treatment | Mother reported symptoms post-treatment |
|---|---|
| Chronic snoring while asleep (every night) | Quiet sleep (no noise heard in room while asleep) |
| Grinding noises while asleep | Quiet sleep |
| Hyperactivity, poor attention, restless behaviour | Better concentration, less inattentiveness |
| Chronic mouth breathing | Notable lips together during daytime |
All procedures were performed in accordance with the ethical standards of the institution and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The mother provided written consent for de-identified information related to this treatment on her child to be used for educational and research purposes.
Discussion
PBM has been shown to be an effective treatment for certain diseases including inflammatory conditions such as chronic pain, to upregulate the immune system, and to aid in wound closure (1-11). PBM is now also being used for sleep breathing disorders; specifically for snoring and, more controversially, for OSA (17-20). This therapy is primarily being delivered by dentists using the Fotona LightWalker laser, and is targeted specifically at the soft palate. The mechanism of action has been studied histologically in animal studies with soft palate tissue appearing to undergo immediate mucosal contraction and keratinization (42,43). Studies also indicate that these effects are not life-long and may begin to taper within three weeks of treatment (42). This may be the reason that “top up” treatments for the treatment of snoring in humans are necessary with the Er:YAG lasers.
POSA is a significant medical problem linked with permanent cognitive functional loss, decreased emotional regulation, and growth stunting if not treated in children (26,36,37,39,44-50). First line treatment for POSA involves surgical excision of tonsils and adenoids (T&A), which brings with it both short- and long-term risks (36-39). As well, some children undergoing T&A surgery for POSA have been shown to require additional treatment even after T&A excision (51-53).
PBM appears to be a potential adjunctive treatment for children suffering from hypertrophic tonsils. However, insufficient research exists into its utility for appropriate patient selection, delivery, and long-term outcomes. Despite this lack of research, this case study appears to indicate PBM is a possible minimally invasive therapy for children with hypertrophic tonsils suffering from symptoms of POSA as noted on the PSQ, at least within the short term. The lack of a pre-intervention and post-intervention Level 1 polysomnogram (PSG), the gold standard for diagnosing POSA, makes any interpretation of the actual effects of PBM on presumed POSA in this child speculative at best. PBM presents a non-painful low risk treatment option for children requiring urgent therapy with symptoms of POSA presenting with hypertrophic tonsils who are unable to see an appropriate physician immediately. For the child in this case report, the reduction in PSQ score of 9/22 (0.409) down to 1/21 (0.048) is a significant drop, indicating the child dropping from high risk of POSA (PSQ pretreatment above cutoff of 0.33) to low risk of POSA as measured by the PSQ (40,41). However, the lack of pre-intervention and post-intervention PSG is a critical limitation in any potential extrapolation of this intervention as a treatment for POSA. The use of PBM may shrink hypertrophic tonsils, immediately opening the airway and allowing for other pediatric airway treatments, such as facial orthopedics/orthodontics and myofunctional therapy, while patients are waiting for other appropriate consultation and evaluation (25,51-57). Clinical research into appropriate patient selection and exclusion criteria, PBM effect duration, and appropriate clinical delivery protocols should be explored.
Conclusions
PBM is a potentially safe and effective treatment for pediatric hypertrophic tonsils in patients presenting with symptoms of POSA as revealed on the PSQ. Further research into this non-invasive painless treatment option is necessary.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/pm-21-18
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pm-21-18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were performed in accordance with the ethical standards of the institution and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The mother provided written consent for de-identified information related to this treatment on her child to be used for educational and research purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cassano P, Petrie SR, Hamblin MR, et al. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 2016;3:031404. [Crossref] [PubMed]
- Cassano P, Tran AP, Katnani H, et al. Selective photobiomodulation for emotion regulation: model-based dosimetry study. Neurophotonics 2019;6:015004. [Crossref] [PubMed]
- Gonzalez-Lima F, Barrett DW. Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci 2014;8:36. [Crossref] [PubMed]
- Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin 2016;6:113-24. [Crossref] [PubMed]
- Keszler A, Lindemer B, Weihrauch D, et al. Red/near infrared light stimulates release of an endothelium dependent vasodilator and rescues vascular dysfunction in a diabetes model. Free Radic Biol Med 2017;113:157-64. [Crossref] [PubMed]
- Kuryliszyn-Moskal A, Kita J, Dakowicz A, et al. The influence of Multiwave Locked System (MLS) laser therapy on clinical features, microcirculatory abnormalities and selected modulators of angiogenesis in patients with Raynaud's phenomenon. Clin Rheumatol 2015;34:489-96. [Crossref] [PubMed]
- Silverman R. Getting athletes back in the game: Low-level laser therapy for sports injuries. Dynamic Chiropract. 2014;32:22-9.
- Liu TC, Cheng L, Su E, et al. Randomized double-blind, and placebo-controlled clinic report of intranasal low-intensity laser therapy on vascular diseases Int J Photoenergy 2012;2012:489713. [Crossref]
- Vallone F, Benedicenti S, Sorrenti E, et al. Effect of diode laser in the treatment of patients with nonspecific chronic low back pain: a randomized controlled trial. Photomed Laser Surg 2014;32:490-4. [Crossref] [PubMed]
- Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 2005;280:4761-71. [Crossref] [PubMed]
- Yadav A, Gupta A. Noninvasive red and near-infrared wavelength-induced photobiomodulation: promoting impaired cutaneous wound healing. Photodermatol Photoimmunol Photomed 2017;33:4-13. [Crossref] [PubMed]
- Kui A, Tisler C, Ciumasu A, et al. Effect of low level laser therapy (LLLT) on muscle pain in temporomandibular disorders – an update of literature Balneo Res J 2020;11:14-9. [Crossref]
- Barolet D, Boucher A. Prophylactic low-level light therapy for the treatment of hypertrophic scars and keloids: a case series. Lasers Surg Med 2010;42:597-601. [Crossref] [PubMed]
- Ezzati K, Fekrazad R, Raoufi Z. The Effects of Photobiomodulation Therapy on Post-Surgical Pain. J Lasers Med Sci 2019;10:79-85. [Crossref] [PubMed]
- Ide Y. Phototherapy for chronic pain treatment. Masui 2009;58:1401-6. [PubMed]
- Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 2013;32:41-52. [PubMed]
- Neruntarat C, Khuancharee K, Shoowit P. Er:YAG laser for snoring: a systemic review and meta-analysis. Lasers Med Sci 2020;35:1231-8. [Crossref] [PubMed]
- Cetinkaya EA, Turker M, Kiraz K, et al. Er:Yag Laser Treatment of Simple Snorers in an Outpatient Setting. ORL J Otorhinolaryngol Relat Spec 2016;78:70-6. [Crossref] [PubMed]
- Frelich H, Ścierski W, Marków M, et al. Minimally invasive erbium laser treatment for selected snorers. Lasers Med Sci 2019;34:1413-20. [Crossref] [PubMed]
- Storchi IF, Parker S, Bovis F, et al. Outpatient erbium:YAG (2940 nm) laser treatment for snoring: a prospective study on 40 patients. Lasers Med Sci 2018;33:399-406. [Crossref] [PubMed]
- Baweja R, Calhoun S, Baweja R, et al. Sleep problems in children. Minerva Pediatr 2013;65:457-72. [PubMed]
- Guilleminault C. Sleep and its disorders in children. Raven Press, New York, 1987.
- Hoban TF. Sleep and its disorders in children. Semin Neurol 2004;24:327-40. [Crossref] [PubMed]
- Lam JC, Mason TB. Treatment of sleep disorders in children. Curr Treat Options Neurol 2007;9:404-13. [Crossref] [PubMed]
- Walter L, Horne R, Nixon G. Treatment of obstructive sleep apnea in children. Clinical Practice 2013;10:519-33. [Crossref]
- Katz ES, D'Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:253-62. [Crossref] [PubMed]
- Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704-12. [Crossref] [PubMed]
- Darrow DH. Surgery for pediatric sleep apnea. Otolaryngol Clin North Am 2007;40:855-75. [Crossref] [PubMed]
- Hoban TF. Sleep disorders in children. Ann N Y Acad Sci 2010;1184:1-14. [Crossref] [PubMed]
- Osman AM, Carter SG, Carberry JC, et al. Obstructive sleep apnea: current perspectives. Nat Sci Sleep 2018;10:21-34. [Crossref] [PubMed]
- Eckert DJ. Phenotypic approaches to obstructive sleep apnoea - New pathways for targeted therapy. Sleep Med Rev 2018;37:45-59. [Crossref] [PubMed]
- Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263-76. [Crossref] [PubMed]
- American Sleep Apnea Association. Sleep apnea treatment options. Available online: https://www.sleepapnea.org/treat/sleep-apnea-treatment-options/. Accessed April 22, 2020
- Veasey SC, Guilleminault C, Strohl KP, et al. Medical therapy for obstructive sleep apnea: a review by the Medical Therapy for Obstructive Sleep Apnea Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 2006;29:1036-44. [Crossref] [PubMed]
- Patil SP, Ayappa IA, Caples SM, et al. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med 2019;15:301-34. [Crossref] [PubMed]
- Contencin P, Guilleminault C, Manach Y. Long-term follow-up and mechanisms of obstructive sleep apnea (OSA) and related syndromes through infancy and childhood. Int J Pediatr Otorhinolaryngol 2003;67:S119-23. [Crossref] [PubMed]
- Tan HL, Gozal D, Kheirandish-Gozal L. Obstructive sleep apnea in children: a critical update. Nat Sci Sleep 2013;5:109-23. [PubMed]
- Chandrakantan A, Musso MF, Floyd T, et al. Pediatric obstructive sleep apnea: Preoperative and neurocognitive considerations for perioperative management. Paediatr Anaesth 2020;30:529-36. [Crossref] [PubMed]
- Capdevila OS, Kheirandish-Gozal L, Dayyat E, et al. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc 2008;5:274-82. [Crossref] [PubMed]
- Chervin RD, Hedger K, Dillon JE, et al. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med 2000;1:21-32. [Crossref] [PubMed]
- Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007;133:216-22. [Crossref] [PubMed]
- Unver T, Aytugar E, Ozturan O, et al. Histological Effects of Er:YAG Laser Irradiation with Snoring Handpiece in the Rat Soft Palate. Photomed Laser Surg 2016;34:321-5. [Crossref] [PubMed]
- Wang Z, Rebeiz EE, Shapshay SM. Laser soft palate "stiffening": an alternative to uvulopalatopharyngoplasty. Lasers Surg Med 2002;30:40-3. [Crossref] [PubMed]
- Schneider HE, Lam JC, Mahone EM. Sleep disturbance and neuropsychological function in young children with ADHD. Child Neuropsychol 2016;22:493-506. [Crossref] [PubMed]
- Cha J, Zea-Hernandez JA, Sin S, et al. The Effects of Obstructive Sleep Apnea Syndrome on the Dentate Gyrus and Learning and Memory in Children. J Neurosci 2017;37:4280-8. [Crossref] [PubMed]
- Gozal D. Obstructive sleep apnea in children: implications for the developing central nervous system. Semin Pediatr Neurol 2008;15:100-6. [Crossref] [PubMed]
- Nachalon Y, Lowenthal N, Greenberg-Dotan S, et al. Inflammation and growth in young children with obstructive sleep apnea syndrome before and after adenotonsillectomy. Mediators Inflamm 2014;2014:146893. [Crossref] [PubMed]
- Operto FF, Precenzano F, Bitetti I, et al. Emotional Intelligence in Children with Severe Sleep-Related Breathing Disorders. Behav Neurol 2019;2019:6530539. [Crossref] [PubMed]
- Smith DL, Gozal D, Hunter SJ, et al. Impact of sleep disordered breathing on behaviour among elementary school-aged children: a cross-sectional analysis of a large community-based sample. Eur Respir J 2016;48:1631-9. [Crossref] [PubMed]
- Xanthopoulos MS, Gallagher PR, Berkowitz RI, et al. Neurobehavioral functioning in adolescents with and without obesity and obstructive sleep apnea. Sleep 2015;38:401-10. [Crossref] [PubMed]
- Guilleminault C, Huang YS, Monteyrol PJ, et al. Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Med 2013;14:518-25. [Crossref] [PubMed]
- Guilleminault C, Huang YS. From oral facial dysfunction to dysmorphism and the onset of pediatric OSA. Sleep Med Rev 2018;40:203-14. [Crossref] [PubMed]
- Huang YS, Hsu SC, Guilleminault C, et al. Myofunctional Therapy: Role in Pediatric OSA. Sleep Med Clin 2019;14:135-42. [Crossref] [PubMed]
- Aroucha Lyra MC, Aguiar D, Paiva M, et al. Prevalence of sleep-disordered breathing and associations with malocclusion in children. J Clin Sleep Med 2020;16:1007-12. [Crossref] [PubMed]
- Camacho M, Chang ET, Song SA, et al. Rapid maxillary expansion for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2017;127:1712-9. [Crossref] [PubMed]
- Villa MP, Rizzoli A, Miano S, et al. Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up. Sleep Breath 2011;15:179-84. [Crossref] [PubMed]
- Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: a 12-year follow-up. Sleep Med 2015;16:933-5. [Crossref] [PubMed]
Cite this article as: Ng ET, Lagravère MO, David A. Photobiomodulation for pediatric hypertrophic tonsils: a clinical case report. Pediatr Med 2021;4:40.


