
Preterm infants born prior to 32 weeks gestation experience more symptoms of gastroesophageal reflux in the first 6 months of life than infants born at later gestational ages
Introduction
Infants born preterm and hospitalized in the neonatal intensive care unit (NICU) are frequently reported to have symptoms associated with gastroesophageal reflux (GER) (1). During hospitalization, preterm infants diagnosed with GER have longer lengths of stay and higher medical costs than infants without GER (1-3). The trajectory of change in symptoms over the first months of life after hospital discharge from the NICU and the influence of gestational age at birth on GER symptomatology during these post-discharge months is not well understood. GER symptoms during this time are important because they are associated with feeding difficulties (4), which may impact the infant’s growth and development. The objective of this study was to describe symptoms of GER across the first 6 months of life in infants based on gestational age at birth. Additionally, factors known at the time of birth were explored for their association to later GER symptoms. We present the following article in accordance with STROBE guidelines for cross-sectional studies (5) (available at http://dx.doi.org/10.21037/pm-20-100).
Methods
This was a descriptive, cross-sectional study of parent-reported symptoms of GER in infants. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the University of North Carolina (IRB number 16-2706), Boston College (IRB numbers 18.087.01 and 19.287.01), and Atrium Health (IRB number 09-19-34E) institutional review boards. Informed consent was taken from all individual participants.
Data were collected and managed using online surveys on the Qualtrics (Provo, UT) platform and REDCap electronic capture tools hosted at Atrium Health (6,7). Participants were recruited from a variety of sites, including infants discharged from the NICUs of Atrium Health Levine Children’s Hospital and University of North Carolina Children’s Hospital; infants cared for in the pediatric primary care clinic and the feeding and swallowing clinic at the University of North Carolina; Researchmatch.org, a national health volunteer registry supported by the National Institutes of Health and the Clinical and Translational Science Award (CTSA) program; Join the Conquest, a health volunteer registry through the CTSA program at the University of North Carolina; Qualtrics respondent panels; online parent support groups; and an informational email sent to faculty, staff, and students at the University of North Carolina at Chapel Hill. Data collection occurred between January 2017 and November 2020. To be eligible for participation, parents had to be at least 18 years old, self-identify as being able to read English, have access to the internet to complete the survey, and have an infant less than 6 months corrected gestational age.
To be included in the full-term infant group, infants had to be ≥37 weeks gestation at birth and the parent had to indicate that the infant did not have any of the following conditions: genetic disorder, cystic fibrosis, congenital diaphragmatic hernia, chronic lung disease, developmental delay, metabolic disorder, epilepsy, hearing or vision impairment, feeding problem, diagnosed food allergy in the infant, or structure abnormality involving the face, mouth, or gastrointestinal tract. To be included in the preterm infant group, the infant had to be <37 weeks gestation at birth.
Variables
Gestational age at birth
The infant’s gestational age at birth and was calculated based on the infant’s date of birth and their expected date of delivery (i.e., due date). Infants were categorized into the following categories: <32 0/7 weeks gestation, 32 0/7–36 6/7 weeks gestation, or ≥37 0/7 weeks gestation (i.e., full term).
Corrected age at time of study
Infants were also categorized by their corrected gestational age at the time of their parent’s participation in the study. These categories were calculated using the infant’s gestational age at birth, date of birth, and date of survey completion. Infants were categorized as 0–2 months (i.e., birth to 2 months 0 days old), 2–4 months (i.e., 2 months 1 day to 4 months 0 days old), or 4–6 months (i.e., 4 months 1 day to 6 months 0 days old).
Factors known at time of birth
Parents were asked several additional questions about the infant’s birth and family history that were used to explore possible contributing factors to later symptoms of GER. Specifically, parents were asked to report the infant’s sex assigned at birth (i.e., male or female), mode of birth (i.e., vaginal delivery or cesarean section), and whether there was a family history (defined as siblings, parents, or grandparents) of food allergies (i.e., yes or no).
Measurement
Symptoms of GER were measured using the Infant Gastroesophageal Reflux Questionnaire-Revised (I-GERQ-R). The I-GERQ-R is a 12-item parent-reported questionnaire about symptoms of GER in the seven days prior to the questionnaire being completed. Individual items were used to calculate a sum total score with a possible range from 0 to 42, with 0 indicating no symptoms of GER and scores increasing with added symptom burden. The I-GERQ-R has adequate psychometric properties, including internal consistency reliability, test-retest reliability, construct validity, and discriminate validity (8-10). Internal consistency reliability of the 12 items on the I-GERQ-R was acceptable in this sample of 582 infants (Cronbach’s alpha =0.75).
Study size
An a priori power analysis [G*Power 3.1.9.7, Düsseldorf, Germany) determined that a sample of at least 17 infants within each gestational age group (i.e, <32 0/7 weeks, 32 0/7–36 6/7 weeks, and ≥37 weeks)] would be sufficient to achieve 95% power with an alpha of .05 (two-tailed) given an effect size of 1.3 for the primary outcome of I-GERQ-R total score. An effect size of 1.3 was chosen based on the average of the standardized mean differences reported in a meta-analysis (11) of seven studies that used the I-GERQ-R (12-18). We also sought to have an approximately equal distribution across age groups (0–2 months, 2–4 months, and 4–6 months). To be included in the analysis, there had to be no missing data on the 12 I-GERQ-R questions.
Statistical methods
All statistical analyses were conducted using IBM SPSS Statistics version 25 (Armonk, New York, USA). To explore whether degree of prematurity was associated with differences in GER symptoms, one-way analysis of variance (ANOVA) was used to determine whether I-GERQ-R total score differed between groups by degree of prematurity. Fisher’s Least Significant Difference (LSD) was used for post-hoc comparisons. To explore whether corrected age at time of study was associated with differences in GER symptoms, taking into account degree of prematurity, univariate general linear models were used, also using Fisher’s LSD for post-hoc comparisons. Within each gestational age group category, ANOVA was used to determine whether changes existed between groups of infants by corrected gestational age at time of study. Finally, to explore the contribution of other factors, each of the following factors was entered into the univariate general linear model separately to identify whether each factor had a significant association with GER symptoms, taking into account degree of prematurity and corrected age at time of study. Statistical significance was defined as P<0.05 for all statistical tests.
Results
Participants
Data from 582 unique infant cases were included in the analysis. Characteristics of the infant sample are included on Table 1. Demographic information about the infants and their families are provided on Table 2. The vast majority of participants (n=574) were located in the United States of America. Of the eight participants not from the United States, there were three from Canada, two from the United Kingdom of Great Britain and Northern Ireland, and one each from Malaysia, Mexico, and Turkey.
Table 1
| < 32 0/7 weeks, n=49 (9%) | 32 0/7–36 6/7 weeks, n=65 (11%) | ≥37 weeks, n=468 (80%) | |
|---|---|---|---|
| Female | 25 (51%) | 36 (55%) | 252 (54%) |
| 0–2 mos | 23 | 17 | 156 |
| 2–4 mos | 21 | 31 | 142 |
| 4–6 mos | 5 | 17 | 170 |
Table 2
| Characteristics | n [%] |
|---|---|
| Respondent’s Relationship to Child (n=575) | |
| Mother | 541 [94] |
| Father | 30 [5] |
| Other Primary Caregiver (e.g., Grandparent) | 4 [1] |
| Family Type (n=574) | |
| Two Parent | 513 [90] |
| One Parent | 49 [8] |
| Other Family Type | 12 [2] |
| Family Income (United States Dollars; n=570) | |
| <$20,000 | 48 [8] |
| $20,000–99,999 | 365 [64] |
| >$100,000 | 157 [28] |
| Child’s Race/Ethnicity (n=575) | |
| American Indian or Alaskan Native | 6 [1] |
| Asian | 25 [4] |
| Black or African American | 43 [8] |
| Hispanic or Latino | 37 [6] |
| White | 373 [65] |
| More than one race | 82 [14] |
| Other | 9 [4] |
| Mode of birth (n=539) | |
| Vaginal | 383 [71] |
| Cesarean section | 156 [29] |
| Family History of Allergy (n=525) | |
| Yes | 158 [27] |
| No | 367 [63] |
Main results
Degree of Prematurity
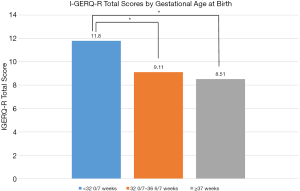
There was a statistically significant difference in I-GERQ-R total score between infants born at different gestational ages (F2,579 = 11.18, P<0.001, η2 =0.04; Figure 1). Infants born <32 0/7 weeks (M =11.8, SD =6.61) had significantly more symptoms of GER than infants born at 32 0/7–36 6/7 weeks (M =9.11, SD =4.55; P<0.01) and infants born ≥37 weeks (M =8.51, SD =4.41; P<0.001). Infants born at 32 0/7–36 6/7 weeks had similar symptoms of GER as infants born at ≥37 weeks (P=0.33).
Corrected age at time of study
Taking into account gestational age at birth, there was a statistically significant difference in I-GERQ-R total scores by corrected age at time of study (F2,577=8.14, P<0.001, η2=0.03; Figure 2). Within the group of infants born at ≥37 weeks, symptoms of GER decreased significantly with increasing corrected age at time of study (F2,465=12.74, P<0.001, η2=0.05). Infants born at ≥37 weeks who were 0–2 months old had more symptoms (M =9.77, SD =4.7) than infants 2–4 months old (M =8.51, SD =4.11; P=0.01) or 4–6 months old (M =7.36, SD =4.08; P<0.001). Infants born at ≥37 weeks who were 2–4 months old had more symptoms that infants 4–6 months old (P=0.02).
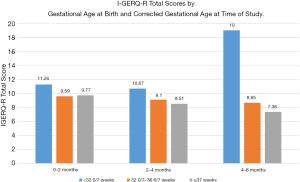
Within the group of infants born at 32 0/7–36 6/7 weeks gestation, there was no significant difference in GER by corrected age at time of study (F2,62=0.18, P=0.84, η2=0.006). Within the group of infants born at <32 0/7 weeks, there a significant difference in symptoms of GER by corrected age at time of study (F2,46=3.73, P=0.03, η2=0.14), but in the opposite direction of change seen in full term infants. For infants born at <32 0/7 weeks gestation, there was no difference in GER symptoms between infants 0–2 months (M =11.26, SD =5.61) and 2–4 months (M =10.67, SD =5.75) corrected age (P=0.76), but infants 4–6 months corrected age had significantly more symptoms (M =19.0, SD =10.65) than those 2–4 months (P=0.01) or 0–2 months old (P=0.02).
Factors known at time of birth
Taking into account gestational age at birth and corrected age at time of study, the infant’s sex was not associated with any difference in symptoms of GER (F1,576 =0.21, P=0.65). Similarly, mode of birth (i.e., vaginal delivery vs. cesarean section) was not associated with any difference in symptoms (F1,533 =0.09, P=0.77), when gestational age at birth and corrected age at time of study were taken into account. Family history of allergies, however, were found to be significantly associated with symptoms of GER (F1,519 = 8.34, P<0.01, η2=0.02) when gestational age at birth and corrected age at time of study were taken into account; infants with a family history of allergy had I-GERQ-R total scores 1.3 points higher than infants without a family history of allergy.
Discussion
In this study, infants born <32 0/7 weeks were found to have more symptoms of GER than infants born at later gestational ages. The pathophysiologic mechanisms for the increased symptoms of GER in these populations of infants are not well understood, but may be related to several factors known to be related to prematurity. There may be developmental alterations in the gastrointestinal tract related to early exposure of the immature gut to microbes (19) or changes related to gastrointestinal injury (e.g., necrotizing enterocolitis) that impact later gastrointestinal functioning (20). Infants born preterm are also known to experience alterations to the gut microbiome (21) that may impact their symptoms of GER. Chronic, toxic stress related to prolonged hospitalization and the frequent, painful events associated with interventions common to newborn intensive care of the infant born prior to 32 weeks, may contribute to inflammation (22) along the gastrointestinal tract and epigenetic changes to glucocorticoid receptor function that may impact the infant’s inflammatory response (23). Infants born prior to 32 weeks also frequently experience respiratory disease and growth faltering, which may require increased feeding volumes and nutritional changes, that may contribute to GER symptoms (24).
Infants born full-term (at ≥37 weeks) showed improvements in GER symptoms with increasing corrected age, which is consistent with data from other studies showing improvements over the first 6 months of life in healthy infants (18,25). Infants born at 32 0/7–36 6/7 weeks, however, did not show improvement, and infants born at <32 0/7 weeks showed worsening symptoms across the first 6 months of age. Although infants born 32 0/7–36 6/7 weeks did not exhibit more symptoms than infants born full-term, they failed to have the same improvement in symptoms with increasing age. Infants born 32 0/7–36 6/7 weeks are at lower risk for long-term morbidity than infants born before 32 weeks gestation; these infants are often not followed as closely after discharge and are an understudied population, but our data indicate that these infants may continue to be at heightened risk for GER.
GER in healthy, full-term infants is thought to result from a combination of a mainly liquid diet, horizontal positioning prior to the ability to sit upright, and the anatomy of the lower esophageal sphincter (24). As the infant approaches six months of life, they typically begin to develop trunk stability and the ability to sit upright. At the same time, the esophagus lengthens (26) so that the lower esophageal sphincter is at the level of the diaphragmatic crura, which provides additional support to maintain tone of the lower esophageal sphincter and prevent reflux of gastrointestinal contents (27). These combined factors result in improvement in GER symptoms as full-term infants approach and move beyond 6 months of age (28).
The mechanisms behind why preterm infants do not have an improvement in symptoms in the same way is not understood. It may be related to delays in development of truncal stability, differences in vagal tone related to the stresses of NICU hospitalization, inflammation along the gastrointestinal tract (29), or differences in the type of nutrition provided. A diet of exclusively human milk is associated with fewer symptoms of GER (30). Infants born preterm are fed human milk for shorter periods of time than infants born full-term (31,32) and are often fed human milk or formula with added calories, protein, or fat to enhance growth (33,34). Additives may increase the osmolality of the human milk or formula (35), which may have an effect on the infant’s digestion and GER symptoms. In some cases, the increased osmolality may decrease symptoms of GER, while in other cases, symptoms may increase. In preterm infants who are receiving human milk, separation of the mother and baby during hospitalization may result in the infant receiving human milk by bottle rather than directly at the breast, which may alter their microbiome (36). The timing of introduction of solid foods, whether it is prior to 4–6 months corrected gestational age or within the range of 4–6 months chronological age, may also impact the developing microbiome (37,38) and symptoms of GER.
In this sample of infants, sex, and mode of birth (i.e., vaginal or cesarean section) were not associated with differences in symptoms of GER, but having a family history of allergy was associated with increased symptoms of GER. The relationship between childhood allergy and GER is documented (39) and family history is a strong predictor of childhood allergy (40). Our findings indicate that, in addition to preterm birth and younger corrected gestational age, a family history of allergy is a factor that may be helpful in identifying infants at particularly high risk for developing significant GER symptoms. The underlying mechanisms for the relationship between family history of allergy and infant GER symptoms requires further study. A family history of allergy may place the infant at higher risk for allergy and it may be that these infants were displaying symptoms of GER as an indicator of early, undiagnosed food allergy.
Limitations
The main limitation of this study was that the sample sizes of preterm infants within each gestational age cohort were relatively small and the data were cross-sectional. Future studies should seek to include larger sample sizes by gestational age cohort and follow these infants longitudinally to provide better data on the trajectory of change over time. Additionally, to be included, participants had to have access to the internet and a device to complete the online survey; while this allowed for accessing a large, geographically diverse sample, it may result in bias within the sample. Finally, 65% of the participants identified their infant as being white. While the proportion of the sample who identified as white was less than that of the US population, where 76.3% of the population identified as white according to 2019 US Census Bureau data (https://www.census.gov/quickfacts/fact/table/US/PST045219), the sample under-represents infants who identify as Black or African American, Asian, and Hispanic or Latino. Interestingly, 14% of the sample identified their infants as being of more than one race, which is higher than that reported in the US Census data. Generalization of these findings should take this limitation into consideration. Future studies should aim to include a more racio-ethnically diverse sample.
Conclusions
Infants born prior to 32 weeks gestation experience more symptoms of GER than infants born at later gestational ages across the first 6 months of life. While full-term infants’ GER symptoms improve, preterm infants, regardless of degree of prematurity, fail to show improvement in symptoms across the first 6 months. In addition to greater degree of prematurity and younger age, a family history of allergy was found to be related to increased symptoms of GER. This study provides important information about the gastrointestinal health of preterm infants during the relatively understudied period of time after neonatal intensive care discharge.
The first year of life after neonatal discharge is a critical time for growth and development and much more research is needed to understand the morbidities associated with preterm birth that may impact their health during this critical time. Additional research involving larger samples of preterm infants are needed to understand the mechanisms for increased GER symptomatology in infants born before 32 weeks gestation. Understanding these mechanisms will allow for development of targeted management strategies. Additionally, research is needed to understand why full-term infants improve in terms of GER symptoms, but preterm infants do not. Understanding the mechanisms for these differences in trajectories of symptoms will not only allow for targeted treatment, but improve our understanding of the ideal time to implement management strategies in preterm infants. Exploration of GER symptoms in preterm and full-term infants beyond 6 months of life will help to elucidate how these trajectories evolve as children continue to mature and their diets change to incorporate solid foods.
Acknowledgments
Funding: This work was supported by research grants from the National Association of Neonatal Nurses (to BP) and from the Association of Women’s Health, Obstetric, and Neonatal Nursing (AWHONN) and Kimberly-Clark (to BP).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Steven M. Barlow) for the series “Neonatal Feeding and Developmental Issues” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE checklist. Available at: http://dx.doi.org/10.21037/pm-20-100
Data Sharing Statement: Available at http://dx.doi.org/10.21037/pm-20-100
Peer Review File: Available at http://dx.doi.org/10.21037/pm-20-100
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-100). The series “Neonatal Feeding and Developmental Issues” was commissioned by the editorial office without any funding or sponsorship. KG is a consultant with Astarte Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the University of North Carolina (IRB number 16-2706), Boston College (IRB numbers 18.087.01 and 19.287.01), and Atrium Health (IRB number 09-19-34E) institutional review boards. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jadcherla SR, Slaughter JL, Stenger MR, et al. Practice variance, prevalence, and economic burden of premature infants diagnosed with GERD. Hospital Pediatrics 2013;3:335-41. [Crossref] [PubMed]
- Khalaf MN, Porat R, Brodsky NL, Bhandari V. Clinical correlations in infants in the neonatal intensive care unit with varying severity of gastroesophageal reflux. J Pediatr Gastroenterol Nutr 2001;32:45-9. [Crossref] [PubMed]
- Ferlauto JJ, Walker MW, Martin MS. Clinically significant gastroesophageal reflux in the at-risk premature neonate: relation to cognitive scores, days in the NICU, and total hospital charges. J Perinatol 1998;18:455-9. [PubMed]
- Hill RR, Park J, Pados BF. Bottle-feeding challenges in preterm-born infants in the first 7 months of life. Global Pediatric Health 2020;7 2333794X20952688.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. [Crossref] [PubMed]
- Harris PA, Taylor R, Minor BLThe REDCap consortium, et al. Building an international community of software platform partners. J Biomed Inform 2019;95:103208 [Crossref] [PubMed]
- Kleinman L, Rothman M, Strauss R, et al. The infant gastroesophageal reflux questionnaire revised: development and validation as an evaluative instrument. Clin Gastroenterol Hepatol 2006;4:588-96. [Crossref] [PubMed]
- Orenstein SR, Cohn JF, Shalaby TM, et al. Reliability and validity of an infant gastroesophageal reflux questionnaire. Clin Pediatr (Phila) 1993;32:472-84. [Crossref] [PubMed]
- Orenstein SR, Shalaby TM, Cohn JF. Reflux symptoms in 100 normal infants: diagnostic validity of the infant gastroesophageal reflux questionnaire. Clinical Pediatrics 1996;35:607-14. [Crossref] [PubMed]
- Smith AB, Fawkes N, Kotze H, et al. Clinically meaningful difference for the infant gastroesophageal questionnaire revised version (I-GERQ-R): A quantitative synthesis. Patient Relat Outcome Meas 2020;11:87-93. [Crossref] [PubMed]
- Vandenplas Y, de Schepper J, Verheyden S, et al. A preliminary report on the efficacy of the Multicare AR-bed in 3-week-3 month-old infants on regurgitation, associated symptoms, and acid reflux. Arch Dis Child 2010;95:26-30. [Crossref] [PubMed]
- Van Howe RS, Storms MR. Gastroesophageal reflux symptoms in infants in a rural population: Longitudinal data over the first six months. BMC Pediatr 2010;10:7. [Crossref] [PubMed]
- Khoshoo V, Dhume P. Clinical response to 2 dosing regimens of lansoprazole in infants with gastroesophageal reflux. J Pediatr Gastroenterol Nutr 2008;46:352-4. [Crossref] [PubMed]
- Neu M, Pan Z, Workman R, et al. Benefits of massage therapy for infants with symptoms of gastroesophageal reflux disease. Biol Res Nurs 2014;16:387-97. [Crossref] [PubMed]
- Orenstein SR, McGowan JD. Efficacy of conservative therapy as taught in the primary care setting for symptoms suggesting infant gastroesophageal reflux. J Pediatr 2008;152:310-4. [Crossref] [PubMed]
- Baldassare ME, Di Mauro A, Pignatelli MC, et al. Magnesium alginate in gastro-esophageal reflux: a randomized multicentre cross-over study in infants. Int J Environ Res Public Health 2020;17:83. [Crossref]
- Campanozzi A, Boccia G, Pensabene L, et al. Prevalence and natural history of gastroesophageal reflux: Pediatric prospective study. Pediatrics 2009;123:779-83. [Crossref] [PubMed]
- Claud EC, Lu L, Anton PM, et al. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci USA 2004;101:7404-8. [Crossref] [PubMed]
- Murthy K, Yanowitz TD, DiGeronimo R, et al. Short-term outcomes for preterm infants with surgical necrotizing enterocolitis. J Perinatol 2014;34:736-40. [Crossref] [PubMed]
- Ho TTB, Groer MW, Kane B, et al. Dichotomous development of the gut microbiome in preterm infants. Microbiome 2018;6:157. [Crossref] [PubMed]
- Vinall J, Grunau RE. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr Res 2014;75:584-7. [Crossref] [PubMed]
- Lester BM, Marsit CJ, Giarraputo J, et al. Neurobehavior related to epigenetic differences in preterm infants. Epigenomics 2015;7:1123-36. [Crossref] [PubMed]
- Pados BF, Davitt ES. Pathophysiology of Gastroesophageal Reflux Disease in Infants and Nonpharmacologic Strategies for Symptom Management. Nurs Womens Health 2020;24:101-14. [Crossref] [PubMed]
- Curien-Chotard M, Jantchou P. Natural history of gastroesophageal reflux in infancy: New data form a prospective cohort. BMC Pediatrics 2020;20:152. [Crossref] [PubMed]
- Moroz SP, Espinoza J, Cumming WA, et al. Lower esophageal sphincter function in children with and without gastroesophageal reflux. Gastroenterology 1976;71:236-41. [Crossref] [PubMed]
- Taeusch HW, Ballard RA, Gleason CA. Avery's diseases of the newborn. 8th ed. Philadelphia: Elsevier Saunders, 2005.
- Pados BF, Yamasaki JT. Symptoms of Gastroesophageal Reflux in Healthy, Full-Term Infants Younger Than 7 Months Old. Nurs Womens Health 2020;24:84-90. [Crossref] [PubMed]
- Filatava EJ, Shelly CE, Thai J, et al. Elevated intestinal inflammation in preterm infants with signs and symptoms of gastroesophageal reflux disease. Biologic Res Nurs 2021. doi:
10.1177/1099800420987888 . [Epub ahead of print].10.1177/1099800420987888 - Andreas NJ, Kampmann B, Le-Doare KM. Human breast milk: A review on its composition and bioactivity. Early Hum Dev 2015;91:629-35. [Crossref] [PubMed]
- Killersreiter B, Grimmer I, Buhrer C, et al. Early cessation of breast milk feeding in very low birthweight infants. Early Hum Dev 2001;60:193-205. [Crossref] [PubMed]
- Bonnet C, Blondel B, Piedvache A, et al. Low breastfeeding continuation to 6 months for very preterm infants: A European multiregional cohort study. Matern Child Nutr 2019;15:e12657 [Crossref] [PubMed]
- Huston RK, Markell AM, McCulley EA, et al. Improving Growth for Infants </=1250 Grams Receiving an Exclusive Human Milk Diet. Nutr Clin Pract 2018;33:671-8. [Crossref] [PubMed]
- Premkumar MH, Massieu LA, Anderson DM, et al. Human Milk Supplements: Principles, Practices, and Current Controversies. Clin Perinatol 2020;47:355-68. [Crossref] [PubMed]
- Levy DS, Osborn E, Hasenstab KA, et al. The effect of additives for reflux or dysphagia management on osmolality in ready-to-feed preterm formula: Practice implications. JPEN J Parenter Enteral Nutr 2019;43:290-7. [Crossref] [PubMed]
- Drell T, Stsepetova J, Simm J, et al. The Influence of Different Maternal Microbial Communities on the Development of Infant Gut and Oral Microbiota. Sci Rep 2017;7:9940. [Crossref] [PubMed]
- Mancabelli L, Tarracchini C, Milani C, et al. Multi-population cohort meta-analysis of human intestinal microbiota in early life reveals the existence of infant community state types (ICSTs). Comput Struct Biotechnol J 2020;18:2480-93. [Crossref] [PubMed]
- Differding MK, Benjamin-Neelon SE, Hoyo C, et al. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol 2020;20:56. [Crossref] [PubMed]
- D'Auria E, Salvatore S, Pozzi E, et al. Cow's Milk Allergy: Immunomodulation by Dietary Intervention. Nutrients 2019;11:1399. [Crossref] [PubMed]
- Kansen HM, Lebbink MA, Mul J, et al. Risk factors for atopic diseases and recurrent respiratory tract infections in children. Pediatr Pulmonol 2020;55:3168-79. [Crossref] [PubMed]
Cite this article as: Pados BF, Briceno G, Feaster V, Gregory K. Preterm infants born prior to 32 weeks gestation experience more symptoms of gastroesophageal reflux in the first 6 months of life than infants born at later gestational ages. Pediatr Med 2021;4:12.

