
Narrative review of muscle weakness and wasting in pediatric critical illness
Introduction
In the past few years, there has been increasing interest in muscle weakness and wasting, and corresponding consequences, in children admitted to the pediatric intensive care unit (PICU) (1-3). Short-term impact of muscle wasting include prolonged mechanical ventilation (MV) requirement, thus increasing PICU length of stay, healthcare costs and resource utilization (2). Long-term consequences are equally concerning, with muscle weakness and wasting extending beyond PICU stay with the potential to affect a child’s physical function and development (4). Functional impairments are one of the types of morbidities observed in the post-intensive care syndrome in children (PICS-p), and these physical limitations can indirectly impact psychological and social function of not only the child, but their siblings and parents as well (5).
However, our understanding of muscle weakness and wasting in critically ill children is still in its infancy—including identification, trajectory, pathophysiology and mechanisms, at risk groups and strategies to overcome muscle weakness and wasting. In adults, there appears to be a better understanding of muscle weakness and wasting in critical illness and recovery. While not completely translatable, adult data can potentially offer insights into PICU muscle weakness and wasting.
The aim of this review is thus to summarize the literature on muscle weakness and wasting in critically ill children with extrapolation of adult data, where potentially applicable in children. We will discuss the tools used to measure muscle changes in critically ill children, and propose future research areas in the study of muscle weakness and wasting in critically ill children.
Methods
Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Embase databases were searched for articles from the earliest available date until 7 June 2020 by a single author (C Ong). The following keywords were used: “skeletal muscle”, “muscle wasting”, “muscle weakness”, “myopathy”, “critical illness” and “intensive care unit”. All full articles written in English, pertaining to critically ill children (0–18 years) were included. In addition, relevant adult literature was included and summarized to provide background on the pathophysiology of muscle wasting in critical illness.
Muscle weakness in critically ill adults
The burden of disability following critical illness has gained attention as long-term morbidities in survivors of critical illness have become apparent. This was described in the seminal studies performed by Herridge et al., where survivors of adult acute respiratory distress syndrome were followed up to 5 years after their intensive care unit (ICU) stay (6,7). Survivors reported impairments in physical function, which persisted at 5 years post illness, and was associated with increased medical costs and inability to return to work. It is now recognized that the effects of critical illness are not confined to the ICU; recovery from the sequelae of critical illness can take years following discharge.
Physical impairment due to critical illness has also been extensively reported in other studies (8,9). Survivors not only experience difficulties returning to work, they also have problems with strength, engaging in extensive physical activity and basic activities such as walking independently (7,8). Lung and cardiac dysfunctions have been suggested as reasons for functional impairment, but these deficits usually resolve after ICU stay and do not appear to be responsible for long-term functional impairment (7,10,11). Herridge et al. found that survivors of acute respiratory distress syndrome attributed their long-term physical limitations to muscle loss and weakness acquired during ICU stay (6). This muscle loss and weakness is now widely studied, and appears to be the cause of significant medical, financial and social burden to ICU survivors and their families (7,12-15).
The weakness responsible for functional impairment occurs in up to 25–33% in patients who are mechanically ventilated for at least 4 to 7 days (14,16), and primarily involves muscle wasting (14,17-19), although in a subset, polyneuropathy may occur (17). However due to the challenges in differentiation and the often co-existence of myopathy and polyneuropathy, an umbrella term “ICU acquired weakness” (ICUAW) is used to describe this phenomenon (20,21). Currently, there is no consensus as to how ICUAW can be diagnosed (22), although a bedside diagnosis of weakness in critically ill patients without an alternate etiology is generally used (21). Manual muscle testing is often performed, most commonly using the Medical Research Council (MRC) muscle strength score (MRC score), which was first validated for use in Guillian-Barre patients (23). This score tests the ability of different muscle groups to overcome varying levels of resistance on a scale of 0 to 5, and a total MRC score of <48 out of 60 has been commonly used as an indicator of ICUAW (21,22).
Muscle weakness and wasting due to critical illness appears to be an increasing and debilitating problem both within and outside the ICU. Besides the long-term functional impairment described above, significant short-term consequences include difficulty in weaning off MV (14,24) and increased risk of mortality (25). Although the exact pathophysiology is unclear, causes of ICU muscle wasting are likely multi-factorial—a combination of critical illness metabolic alterations and ICU therapy (21). Muscle mass is determined by the net balance between protein breakdown and synthesis, which are regulated by catabolic and anabolic pathways respectively (26,27). In most healthy adults, protein breakdown and synthesis are balanced and muscle mass is maintained. Critically ill adults experience elevated muscle breakdown early in the disease course (13,28), and results in a negative protein balance which improves over time (29,30). However, post-discharge data has demonstrated that muscle does not always return to baseline size, indication possible long-term muscle deficits (31).
Muscle weakness and wasting in critically ill children
Muscle weakness has also been reported in critically ill children in the past two decades (32-38). Case reports illustrate clinical observations of flaccid paralysis after weaning of sedatives or failure to extubate from MV, which occurred after 7 to 25 days of PICU stay (33-38). Several risk factors of muscle weakness and wasting have been proposed (39,40). The use of neuromuscular blockade may be a possible risk factor for ICUAW in children, as they were used in all of the case reports and also suggested as the main cause in several reports (32,35,41). Organ dysfunction score was also higher in children with muscle weakness compared to those without (38). These risk factors are similar to risk factors that have been reported in adult ICU muscle weakness (14,39,40), suggesting similar underlying pathophysiology of ICUAW in adult and children. These include a combination of myopathy and polyneuropathy as a result of disuse and immobilization, inflammation, altered circulating hormones, malnutrition and medication use (20,22). Of note, this phenomenon is different from sarcopenia in older adults, which involves an age-related decline in skeletal muscle mass and function (42).
The prevalence of muscle weakness in critically ill children appears to remains low compared to critically ill adults. In a prospective cohort study, Banwell et al. studied the incidence of muscle weakness children admitted to a general PICU for >24 hours over a period of 1 year (n=830). Muscle weakness was defined using a cut-off of MRC grade ≤4 in any muscle group, reduced or absent tendon reflexes and an inability to wean from MV as definitions of muscle weakness (32). The authors reported a prevalence of 1.7% (14/830), which is lower than the median prevalence reported across adult critically care studies of 30% in general ICU cohorts, and 64% in adult sepsis patients (22).
Part of the difference in adults versus children may be the difficulty in muscle strength testing in critically ill children. Siu et al. attempted to measure the weekly MRC sum score in critically ill children (43). In a cohort of 33 patients aged 1.1 to 16.1 years, the authors found that the MRC tests could not be completed in almost half of the patients. Aside from patients being sedated or comatose, reasons for non-completion included difficulties in understanding, lack of cooperation and poor neurological status, especially in younger patients.
This demonstrates the need for non-volitional, objective measures to detect muscle weakness and wasting in critically ill children, to be able to better identify and characterize the problem. An option for this is the measurement of body composition and muscle during critical illness. With increasing study on muscle changes in adults during critical illness, interest in muscle and body composition changes in critically ill children have also increased. To date, various methods have been used to determine muscle and body composition in critically ill children (Table 1).
Table 1
| Methodology | Brief description | Advantages | Disadvantages |
|---|---|---|---|
| Medical Research Council (MRC) sum score | Used to gauge strength of limb muscles in relation to the assessor’s resistance | Performed at bedside | Subjective |
| No equipment necessary | Requires children to be able to understand instructions and cooperate with measurements | ||
| Skinfolds and circumference | Skinfolds used to measure fat size and overall body fat percentage. Triceps skinfolds often measured together with arm circumference to determine fat and muscle stores | Bedside measurement | Significant inter/intra-rater variability |
| Inexpensive tools required | Accuracy also limited by edema, which is common in critically ill children | ||
| Bioelectrical impedance analysis | Sends a weak electrical current through the body, and using values of resistance and reactance, provides an indicator of fat and fat-free mass | Bedside measurement, easy to administer | Equations to estimate fat and fat-free mass are inaccurate in conditions of fluid overload |
| Objective | Does not inform on distribution of muscle or fat throughout the body | ||
| Ultrasonography | Can be used to assess muscle cross-sectional area, thickness, echogenicity as well as fat thickness. Muscle groups studied: upper and lower limb muscles, diaphragm | Bedside measurement | Ultrasound machine required |
| Objective | Measurements can be operator dependent | ||
| Computed tomography (CT) and magnetic resonance imaging (MRI) | Imaging methods that use specialized machines to detect specific body tissue components. Muscle and fat size can be measured on each image to provide information on fat and muscle size | Gold standard for identification of specific fat and muscle components | Expensive, specialized manpower and equipment required |
| CT scans associated with radiation | |||
| May require sedation for accurate measurement |
Muscle wasting demonstrated by various measurement methodologies
Arm muscle circumference
Traditional anthropometric measurements have included the use of mid-upper arm muscle circumference and triceps skinfold thickness, which can be used to calculate the upper arm muscle circumference and muscle area (44). This method is non-invasive, fast to administer and requires only simple measurement tools such as a tape measure and skinfold calipers.
Zamberlan et al. monitored mid-upper arm circumference and triceps skinfold thickness in critically ill children (n=90) on admission and after a week (45). There was an overall reduction in arm circumference, which was attributed to a decrease in triceps skinfold thickness but not arm muscle circumference. Similar findings were reported by Hulst et al., who monitored mid upper arm circumference in critically ill children from PICU admission to discharge (46). In 93 children, during their PICU stay, significant decreases in mid upper arm circumference and triceps skinfold thickness were observed, but changes in corresponding arm muscle circumference were not reported.
Skinfold measurements are subject to inter-rater differences, which can limit the accuracy of longitudinal changes (47). In addition, edema can affect the accuracy of arm muscle area and skinfolds measurements, which can limit its utility in children who experience significant fluid shifts (48).
Computed tomography (CT) and magnetic resonance imaging (MRI)
CT and MRI scans are considered one of the most accurate methods in visualizing and differentiating between different body tissue components (49,50). Using specialized software and established greyscale thresholds for various tissue components (e.g., skeletal muscle, visceral adipose tissue), skeletal muscle and various adipose tissue components can be measured from MRI and CT images. Our group has used these imaging methods to assess skeletal muscle mass and adipose tissue size at PICU admission (51). Using imaging data from 92 children, we found that higher skeletal muscle and adipose tissue size at PICU admission was associated with functional impairment that persisted to hospital discharge (51). However, CT and MRI scans have not been used to study longitudinal skeletal muscle changes in critically ill children. Part of this reason is the that CT and MRI imaging is rarely done in critically ill children due to the radiation involved in CT scans, as well as the need for specialized equipment and manpower. The low frequency of measurements thus limits the utility of these imaging methods to assess skeletal muscle changes in pediatric critical illness.
Ultrasonography
Muscle ultrasonography in critically ill children has gained recent interest after the use of this method to identify muscle wasting in critically ill adults (52). However, muscle imaging using ultrasound has been used for over two decades in monitoring of changes in neuromuscular diseases such as spinal muscular atrophy and Duchenne muscular dystrophy (53,54).
Limb muscle
Limb muscles play a large role in movement and autonomy, and thus have been monitored in muscle disorders (55-58). In pediatric neuromuscular disease, ultrasounds demonstrate decreasing limb muscle thickness as well as an increase in muscle echogenicity, an indication of progression of disease (56,59). Muscle size and echogenicity measurements have both been shown to correlate with physical function and strength (55,56,59).
In critically ill children, both upper and lower limb muscles have been studied. The most commonly studied muscle has been the quadriceps muscle (Figure 1) (2,3,60), partly due to ease and accessibility of measurement in the immobile, supine state during critical illness. The quadriceps muscles also play an important role in mobility, which is one aspect of physical function that has been shown to be impaired in critically ill adults (7). Other limbs measured include the biceps brachii and tibialis anterior (2).
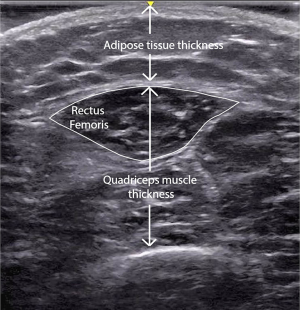
Overall, ultrasounds demonstrate decreases in muscle size during critical illness. Adults have reported an average decrease in quadriceps muscle size of 2% to 3% per day during critical illness (13,61). Two studies have reported similar rates of muscle wasting in critically ill children. In a dual-center study, Valla et al. found a decrease in quadriceps thickness of 9.8% [interquartile range (IQR), 0–13.3%] on day 5 of PICU stay, while Johnson et al. reported a decrease in quadriceps thickness of 1.5% per day (2). In comparison, Johnson et al. found that there were no significant decreases in biceps or tibialis muscles, indicating differences in vulnerability of muscle groups to atrophy during critical illness, and emphasizing the effects of critical illness (2). Factors associated with loss of quadriceps muscles in bivariate analyses included age >1 year, traumatic brain injury and greater body mass index, although these did not remain significant in the final multiple regression model.
Diaphragm muscle
Ultrasound of the diaphragm muscle has been recently studied to understand the rate of diaphragm atrophy, as well as to predict extubation success. Diaphragm ultrasound is most commonly performed between the right anterior and the mid-axillary lines, at the intercostal space between the eighth or the tenth ribs (62-64). Overall rates of diaphragm atrophy in children range from approximately 2.0% to 3.4% per day (2,62,64), lower than rates of 6% to 7.5% reported in adults (65,66). In both children and adults, rates of diaphragm atrophy are the fastest in the first 2 to 3 days, tapering off in subsequent days (62,67). One factor that has been reported to be associated with diaphragm atrophy was the use of neuromuscular blockade, although this was not consistent across studies (2,64).
Diaphragm thickening fraction (DTF), which is commonly measured using M-mode ultrasound, has been used to predict extubation success (62,63). DTF is calculated using the equation [(diaphragm end-inspiratory thickness – end-expiratory thickness) ÷ end-expiratory thickness]. In critically ill children, a DTF cut-off of ≥17% and ≥21% have been reported to predict extubation success in mechanically ventilated children (62,63).
One limitation of ultrasonography is its highly operator-dependent nature, implying that accuracy of measurements can depend on the person performing the measurement (68). Clear protocols and repeated training as well as inter-operator reliability testing would help in improving accuracy and reducing human error in measurements (69,70).
Bioelectrical impedance analysis (BIA)
BIA utilizes electrical currents to inform body fluid status and composition. Based on the respective resistance (R) and reactance (Xc) of current speeds in different medium, BIA is capable of detecting the total body water (71). Resistance and reactance values can also be put into pre-established equations to estimate body fat and fat-free mass components (71). BIA measurements are non-invasive, easy to administer and relatively inexpensive, making it ideal for the PICU setting. However, BIA is subject to fluid shifts—a common occurrence in critically ill patients, which limits the accuracy of the equations used to estimate body composition, and thus the accuracy in informing changes in body composition during critical illness.
With the inaccuracies of fat and fat-free mass estimations during periods of fluid shifts, researchers have shifted towards the use of raw data of resistance and reactance, which are less influenced by fluid status compared to equations used to estimate fat and fat-free mass (72). Resistance and reactance can then be used to calculate phase angle using the equation: arctangent (Xc ÷ R) × (180 ÷ π). Phase angle has been used to gauge the general health of a cell, and an indirectly, muscle mass (73,74). In critically ill adults, several observational studies have reported that lower phase angle on admission was associated with mortality (75-78). Various cut-offs of phase angle reported to be predictive of mortality include <3.49° to <4.8° (77,78). Importantly, Looijaard et al. described a decrease in phase angle, albeit non-significant, in a small cohort (n=15) of critically ill adults (79). This study also reported a correlation between greater protein intake and increases in phase angle, although this could have been confounded by other non-nutritive factors fluid balance.
BIA-derived resistance, reactance and phase angle has also been studied in critically ill children. In a mixed PICU cohort of 247 patients, Zamberlan et al. demonstrated that a phase angle on admission ≤2.8° was associated with higher risk of mortality and a longer PICU stay (80). The authors also found that this phase angle cut-off was able to differentiate between those with a mid-upper arm circumference ≤5th percentile for age, suggesting phase angle as a possible alternative method to identify low nutritional status in critically ill children.
To understand longitudinal BIA changes, Azevedo et al. conducted BIA measurements in a general PICU cohort of 332 children requiring MV at admission and discharge (72). The authors found that reactance and resistance generally increased from 48 hours of PICU admission to PICU discharge, with a greater increase in reactance compared to resistance and an overall increase in phase angle. The authors also found that survival was generally associated with an increase in resistance from PICU admission to discharge, while in non-survivors there was a trend of decrease in either resistance or reactance.
These studies suggest potential for the use of resistance, reactance and phase angle in predicting outcomes in critically ill children. However, how these parameters correlate with nutritional intake and body composition during critical illness remains unclear. In addition, before BIA can be used for body composition analysis in critically ill children, it requires further study, including ensuring that the measurements are able to account for gender, age and ethnic differences in body composition (81).
Understanding muscle wasting and areas of future research
Relationship between muscle and function
While several studies have demonstrated muscle loss in critically ill children (2,3), none have yet correlated muscle changes with physical function in survivors of pediatric critical illness. In adults, recovery from ICU muscle weakness can take time, and physical impairments are seen up to 5 years post critical illness in adults (7). In PICU functional outcome studies, there is evidence that the impairment can also be prolonged (82,83). In the cohort study (n=830) eluded to earlier on the prevalence of muscle weakness in critically ill children, muscle weakness persisted in majority (89%) of the patients at 3 months after discharge, with reported poor physical endurance at 18 months post-discharge in one of the patients (32). Case reports of PICU muscle weakness during also described children suffering from prolonged impairments of certain areas of function despite strength recovery. For example, an 18-month-old girl reported being easily fatigued after strength recovery at 5 months (84), while another patient (21 months) experienced developmental delay including motor delay after strength recovery at 16 months (33). Although this motor delay might not necessarily be due to muscle deficits, they have been reported in children with liver-failure associated muscle wasting, which improved with restoration of weight and muscle mass (85). Determining recovery in children requires not just a return to baseline, but also a catch-up to their peers. This may differ depending on the developmental age and plasticity of the children. Thus, assessing age-appropriate functions in PICU survivors is important to avoid overlooking functional disabilities during the growing ages.
Nutritional and rehabilitative strategies
The evidence for nutritional strategies in improving muscle wasting and physical function remains unclear. In adults, while observational studies have reported reduced muscle wasting with greater energy adequacy (86,87), nutritional interventions have failed to translate to improvements in muscle mass in randomized controlled trials (RCTs). For example, in the Early versus Late Parenteral Nutrition in Critically Ill adults (EPaNIC) RCT, early parenteral protein and energy provision did not ameliorate muscle wasting or result in better physical function, and instead was associated with more muscle weakness and slower recovery (88,89). The Early Versus Delayed Enteral Feeding (EDEN) trial also did not find a difference in physical function between trophic and full enteral feeding in critically ill adults in the first week of ICU stay (90). Individualized, targeted nutrition did not result in better physical quality of life compared to standard of care in the Early Goal-Directed Nutrition in ICU Patients (EAT-ICU) trial (91). A possible explanation for this may be an impaired mitochondrial function and dysregulation of skeletal muscle bioenergetics, as intramuscular adenosine triphosphate content and substrates were found to correlate with muscle loss in critically ill adults (92). These trials collectively suggest the need for further considerations with regards to the timing, substrate and amount of feeding in critical illness.
Early mobilization (EM) is another aspect of care that has been studied in reducing muscle weakness and wasting. In critically ill adults, EM helps to improve function in the adult ICU (93), but there is a lack of consensus and perceived acceptable thresholds for safety guidelines of early rehabilitation in critically ill children (94). While these definitions of EM and safety thresholds differ across units, rehabilitation has generally shown to be safe within the PICU (95). A recent point-prevalence study of physical rehabilitation efforts within the PICUs in the United States demonstrated that children with better pre-PICU function are less likely to receive rehabilitation compared to children of poor pre-PICU function (96), although these children have shown to be at greater risk for functional impairment post-PICU stay (82,97). However, the impact of early rehabilitation on physical function post-PICU stay has not yet been demonstrated. Choong et al. conducted a pilot trial evaluating in-bed cycling in PICU patients, but did not observe a difference in physical function in the intervention group (98).
Research on nutrition and rehabilitation during pediatric critical illness in relation to muscle wasting and functional outcomes is still in its infancy. Extrapolation of adult evidence to the pediatric population must be done with caution, and be respectful of the differences in protein homeostatic responses between adults and children. Interventions that may not have been effective in adults may have different effect on children. For example, feeding has been shown to be highly anabolic in younger children, especially neonates, compared to adults (99). Trials on both nutrition and early rehabilitation are needed in critically ill children to determine its effect on muscle wasting and functional outcomes. Further, it may be important to understand the synergistic combination of nutrition and exercise during critical illness, as is being done in adults in the Nutrition and Exercise in Critical Illness (NEXIS) trial (100).
Conclusions
While evidence demonstrates of muscle weakness and wasting in critically ill children, there is insufficient understanding of the pathophysiology, risk factors and long-term consequences on child function and development. Incorporating non-invasive, objective, bedside methods such as ultrasonography and BIA in the assessment of muscle and body composition changes together with measurement of function and development may help overcome this. Research efforts addressing these can benefit the understanding of the PICS-p.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyvonne Tume, Frederic Valla and Sascha Verbruggen) for the series “Nutrition in the Critically Ill Child” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-83). The series “Nutrition in the Critically Ill Child” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tume LN, Valla FV, Floh AA, et al. Priorities for Nutrition Research in Pediatric Critical Care. JPEN J Parenter Enteral Nutr 2019;43:853-62. [Crossref] [PubMed]
- Johnson RW, Ng KWP, Dietz AR, et al. Muscle atrophy in mechanically-ventilated critically ill children. PLoS One 2018;13:e0207720. [Crossref] [PubMed]
- Valla FV, Young DK, Rabilloud M, et al. Thigh Ultrasound Monitoring Identifies Decreases in Quadriceps Femoris Thickness as a Frequent Observation in Critically Ill Children*. Pediatr Crit Care Med 2017;18:e339-47. [Crossref] [PubMed]
- Senna S, Ong C, Ng ZM, et al. Long-Term Morbidities in Children with Critical Illness: Gaps and Opportunities. Ann Acad Med Singap 2018;47:291-337. [PubMed]
- Manning JC, Pinto NP, Rennick JE, et al. Conceptualizing Post Intensive Care Syndrome in Children—The PICS-p Framework*. Pediatr Crit Care Med 2018;19:298-300. [Crossref] [PubMed]
- Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348:683-93. [Crossref] [PubMed]
- Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293-304. [Crossref] [PubMed]
- van der Schaaf M, Dettling DS, Beelen A, et al. Poor functional status immediately after discharge from an intensive care unit. Disabil Rehabil 2008;30:1812-8. [Crossref] [PubMed]
- Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Depressive Symptoms and Impaired Physical Function after Acute Lung Injury. Am J Respir Crit Care Med 2012;185:517-24. [Crossref] [PubMed]
- Cooper AB, Ferguson ND, Hanly PJ, et al. Long-term follow-up of survivors of acute lung injury: lack of effect of a ventilation strategy to prevent barotrauma. Crit Care Med 1999;27:2616-21. [Crossref] [PubMed]
- Elliott VJ, Rodgers DL, Brett SJ. Systematic review of quality of life and other patient-centred outcomes after cardiac arrest survival. Resuscitation 2011;82:247-56. [Crossref] [PubMed]
- Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:538-44. [Crossref] [PubMed]
- Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591-600. Erratum in: JAMA 2014 Feb 12;311(6):625. Padhke, Rahul [corrected to Phadke, Rahul]. [Crossref] [PubMed]
- De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288:2859-67. [Crossref] [PubMed]
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med 2012;40:502-9. [Crossref] [PubMed]
- de Letter MA, Schmitz PI, Visser LH, et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med 2001;29:2281-6. [Crossref] [PubMed]
- Hermans G, De Jonghe B, Bruyninckx F, et al. Clinical review: Critical illness polyneuropathy and myopathy. Crit Care 2008;12:238. [Crossref] [PubMed]
- Trojaborg W, Weimer LH, Hays AP. Electrophysiologic studies in critical illness associated weakness: myopathy or neuropathy--a reappraisal. Clin Neurophysiol 2001;112:1586-93. [Crossref] [PubMed]
- Koch S, Spuler S, Deja M, et al. Critical illness myopathy is frequent: accompanying neuropathy protracts ICU discharge. J Neurol Neurosurg Psychiatry 2011;82:287-93. [Crossref] [PubMed]
- Schweickert WD, Hall J. ICU-acquired weakness. Chest 2007;131:1541-9. [Crossref] [PubMed]
- Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 2009;37:S299-308. [Crossref] [PubMed]
- Fan E, Cheek F, Chlan L, et al. An Official American Thoracic Society Clinical Practice Guideline: The Diagnosis of Intensive Care Unit–acquired Weakness in Adults. Am J Respir Crit Care Med 2014;190:1437-46. [Crossref] [PubMed]
- Kleyweg RP, van der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve 1991;14:1103-9. [Crossref] [PubMed]
- Garnacho-Montero J, Amaya-Villar R, García-Garmendía JL, et al. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients*. Crit Care Med 2005;33:349-54. [Crossref] [PubMed]
- Ali NA, O'Brien JM Jr, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 2008;178:261-8. [Crossref] [PubMed]
- Mitch WE, Goldberg AL. Mechanisms of Muscle Wasting — The Role of the Ubiquitin–Proteasome Pathway. N Engl J Med 1996;335:1897-905. [Crossref] [PubMed]
- Millward DJ, Garlick PJ, Stewart RJ, et al. Skeletal-muscle growth and protein turnover. Biochem J 1975;150:235-43. [Crossref] [PubMed]
- Wollersheim T, Woehlecke J, Krebs M, et al. Dynamics of myosin degradation in intensive care unit-acquired weakness during severe critical illness. Intensive Care Med 2014;40:528-38. [Crossref] [PubMed]
- Rennie MJ. Muscle protein turnover and the wasting due to injury and disease. Br Med Bull 1985;41:257-64. [Crossref] [PubMed]
- Gamrin-Gripenberg L, Sundström-Rehal M, Olsson D, et al. An attenuated rate of leg muscle protein depletion and leg free amino acid efflux over time is seen in ICU long-stayers. Critical Care 2018;22:13. [Crossref] [PubMed]
- Dos Santos C, Hussain SN, Mathur S, et al. Mechanisms of Chronic Muscle Wasting and Dysfunction after an Intensive Care Unit Stay. A Pilot Study. Am J Respir Crit Care Med 2016;194:821-30. [Crossref] [PubMed]
- Banwell BL, Mildner RJ, Hassall AC, et al. Muscle weakness in critically ill children. Neurology 2003;61:1779-82. [Crossref] [PubMed]
- Heckmatt JZ, Pitt MC, Kirkham F. Peripheral neuropathy and neuromuscular blockade presenting as prolonged respiratory paralysis following critical illness. Neuropediatrics 1993;24:123-5. [Crossref] [PubMed]
- Chetaille P, Paut O, Fraisse A, et al. Acute myopathy of intensive care in a child after heart transplantation. Can J Anaesth 2000;47:342-6. [Crossref] [PubMed]
- Benzing G, Iannaccone ST, Bove KE, et al. Prolonged myasthenic syndrome after one week of muscle relaxants. Pediatr Neurol 1990;6:190-6. [Crossref] [PubMed]
- Sheth RD, Pryse-Phillips WEM, Riggs JE, et al. Critical illness neuromuscular disease in children manifested as ventilatory dependence. J Pediatr 1995;126:259-61. [Crossref] [PubMed]
- Tabarki B, Coffiniéres A, Van Den Bergh P, et al. Critical illness neuromuscular disease: clinical, electrophysiological, and prognostic aspects. Arch Dis Child 2002;86:103-7. [Crossref] [PubMed]
- Vondracek P, Bednarik J. Clinical and electrophysiological findings and long-term outcomes in paediatric patients with critical illness polyneuromyopathy. Eur J Paediatr Neurol 2006;10:176-81. [Crossref] [PubMed]
- Griffin D, Fairman N, Coursin D, et al. Acute myopathy during treatment of status asthmaticus with corticosteroids and steroidal muscle relaxants. Chest 1992;102:510-4. [Crossref] [PubMed]
- Hirano M, Ott BR, Raps EC, et al. Acute quadriplegic myopathy: a complication of treatment with steroids, nondepolarizing blocking agents, or both. Neurology 1992;42:2082-7. [Crossref] [PubMed]
- Geller TJ, Kaiboriboon K, Fenton GA, et al. Vecuronium-associated axonal motor neuropathy: a variant of critical illness polyneuropathy? Neuromuscul Disord 2001;11:579-82. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Siu K, Al-Harbi S, Clark H, et al. Feasibility and Reliability of Muscle Strength Testing in Critically Ill Children. J Pediatr Intensive Care 2015;4:218-24. [Crossref] [PubMed]
- Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr 1981;34:2540-5. [Crossref] [PubMed]
- Zamberlan P, Delgado AF, Leone C, et al. Nutrition Therapy in a Pediatric Intensive Care Unit. JPEN J Parenter Enteral Nutr 2011;35:523-9. [Crossref] [PubMed]
- Hulst J, Joosten K, Zimmermann L, et al. Malnutrition in critically ill children: from admission to 6 months after discharge. Clin Nutr 2004;23:223-32. [Crossref] [PubMed]
- Kispert CP, Merrifield HH. Interrater reliability of skinfold fat measurements. Phys Ther 1987;67:917-20. [Crossref] [PubMed]
- Grippa RB, Silva PS, Barbosa E, et al. Nutritional status as a predictor of duration of mechanical ventilation in critically ill children. Nutrition 2017;33:91-5. [Crossref] [PubMed]
- Rössner S, Bo WJ, Hiltbrandt E, et al. Adipose tissue determinations in cadavers--a comparison between cross-sectional planimetry and computed tomography. Int J Obes 1990;14:893-902. [PubMed]
- Abate N, Burns D, Peshock RM, et al. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res 1994;35:1490-6. [Crossref] [PubMed]
- Ong C, Lee JH, Senna S, et al. Body Composition and Acquired Functional Impairment in Survivors of Pediatric Critical Illness. Crit Care Med 2019;47:e445-53. [Crossref] [PubMed]
- Ong C, Lee JH, Leow MKS, et al. Skeletal Muscle Ultrasonography in Nutrition and Functional Outcome Assessment of Critically Ill Children: Experience and Insights From Pediatric Disease and Adult Critical Care Studies. JPEN J Parenter Enteral Nutr 2017;41:1091-9. [Crossref] [PubMed]
- Pillen S, Verrips A, van Alfen N, et al. Quantitative skeletal muscle ultrasound: Diagnostic value in childhood neuromuscular disease. Neuromuscul Disord 2007;17:509-16. [Crossref] [PubMed]
- Brockmann K, Becker P, Schreiber G, et al. Sensitivity and specificity of qualitative muscle ultrasound in assessment of suspected neuromuscular disease in childhood. Neuromuscul Disord 2007;17:517-23. [Crossref] [PubMed]
- Jacobs J, Jansen M, Janssen H, et al. Quantitative muscle ultrasound and muscle force in healthy children: a 4-year follow-up study. Muscle Nerve 2013;47:856-63. [Crossref] [PubMed]
- Jansen M, van Alfen N, Nijhuis van der Sanden MWG, et al. Quantitative muscle ultrasound is a promising longitudinal follow-up tool in Duchenne muscular dystrophy. Neuromuscul Disord 2012;22:306-17. [Crossref] [PubMed]
- Scholten RR, Pillen S, Verrips A, et al. Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve 2003;27:693-8. [Crossref] [PubMed]
- Midorikawa T, Ohta M, Hikihara Y, et al. Prediction and validation of total and regional skeletal muscle volume using B-mode ultrasonography in Japanese prepubertal children. Br J Nutr 2015;114:1209. [Crossref] [PubMed]
- Ng KW, Connolly AM, Zaidman CM. Quantitative muscle ultrasound measures rapid declines over time in children with SMA type 1. J Neurol Sci 2015;358:178-82. [Crossref] [PubMed]
- Fivez T, Hendrickx A, Van Herpe T, et al. An Analysis of Reliability and Accuracy of Muscle Thickness Ultrasonography in Critically Ill Children and Adults. JPEN J Parenter Enteral Nutr 2016;40:944-9. [Crossref] [PubMed]
- Parry SM, El-Ansary D, Cartwright MS, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care 2015;30:1151.e9-14. [Crossref] [PubMed]
- Lee EP, Hsia SH, Hsiao HF, et al. Evaluation of diaphragmatic function in mechanically ventilated children: An ultrasound study. PLoS One 2017;12:e0183560. [Crossref] [PubMed]
- Xue Y, Zhang Z, Sheng CQ, et al. The predictive value of diaphragm ultrasound for weaning outcomes in critically ill children. BMC Pulm Med 2019;19:270. [Crossref] [PubMed]
- Glau CL, Conlon TW, Himebauch AS, et al. Progressive Diaphragm Atrophy in Pediatric Acute Respiratory Failure. Pediatr Crit Care Med 2018;19:406-11. [Crossref] [PubMed]
- Grosu HB, Lee YI, Lee J, et al. Diaphragm Muscle Thinning in Patients Who Are Mechanically Ventilated. Chest 2012;142:1455-60. [Crossref] [PubMed]
- Zambon M, Beccaria P, Matsuno J, et al. Mechanical Ventilation and Diaphragmatic Atrophy in Critically Ill Patients: An Ultrasound Study. Crit Care Med 2016;44:1347-52. [Crossref] [PubMed]
- Schepens T, Verbrugghe W, Dams K, et al. The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Critical Care 2015;19:422. [Crossref] [PubMed]
- Ohrndorf S, Naumann L, Grundey J, et al. Is Musculoskeletal Ultrasonography an Operator-Dependent Method or a Fast and Reliably Teachable Diagnostic Tool? Interreader Agreements of Three Ultrasonographers with Different Training Levels. Int J Rheumatol 2010;2010:164518. [Crossref] [PubMed]
- Tillquist M, Kutsogiannis DJ, Wischmeyer PE, et al. Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J Parenter Enteral Nutr 2014;38:886-90. [Crossref] [PubMed]
- Zaidman CM, Wu JS, Wilder S, et al. Minimal training is required to reliably perform quantitative ultrasound of muscle. Muscle Nerve 2014;50:124-8. [Crossref] [PubMed]
- Lukaski HC, Johnson PE, Bolonchuk WW, et al. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr 1985;41:810-7. [Crossref] [PubMed]
- Azevedo ZM, Moore DC, de Matos FA, et al. Bioelectrical impedance parameters in critically ill children: importance of reactance and resistance. Clin Nutr 2013;32:824-9. [Crossref] [PubMed]
- Máttar JA. Application of total body bioimpedance to the critically ill patient. Brazilian Group for Bioimpedance Study. New Horiz 1996;4:493-503. [PubMed]
- Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol 2002;86:509-16. [Crossref] [PubMed]
- Kuchnia A, Earthman C, Teigen L, et al. Evaluation of Bioelectrical Impedance Analysis in Critically Ill Patients: Results of a Multicenter Prospective Study. JPEN J Parenter Enteral Nutr 2017;41:1131-8. [Crossref] [PubMed]
- Lee YH, Lee JD, Kang DR, et al. Bioelectrical impedance analysis values as markers to predict severity in critically ill patients. J Crit Care 2017;40:103-7. [Crossref] [PubMed]
- Thibault R, Makhlouf AM, Mulliez A, et al. Fat-free mass at admission predicts 28-day mortality in intensive care unit patients: the international prospective observational study Phase Angle Project. Intensive Care Med 2016;42:1445-53. [Crossref] [PubMed]
- Stapel SN, Looijaard WGPM, Dekker IM, et al. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur J Clin Nutr 2018;72:1019-25. [Crossref] [PubMed]
- Looijaard WGPM, Denneman N, Broens B, et al. Achieving protein targets without energy overfeeding in critically ill patients: A prospective feasibility study. Clin Nutr 2019;38:2623-31. [Crossref] [PubMed]
- Zamberlan P. Bioelectrical Impedance Phase Angle and Morbidity and Mortality in Critically Ill Children. Nutr Clin Pract 2019;34:163-71. Erratum in: Nutr Clin Pract 2020 Apr;35(2):366. doi: 10.1002/ncp.10415. Epub 2019 Oct 13. [Crossref] [PubMed]
- Kyle UG, Earthman CP, Pichard C, et al. Body composition during growth in children: limitations and perspectives of bioelectrical impedance analysis. Eur J Clin Nutr 2015;69:1298-305. [Crossref] [PubMed]
- Choong K, Fraser D, Al-Harbi S, et al. Functional Recovery in Critically Ill Children, the “WeeCover” Multicenter Study. Pediatr Crit Care Med 2018;19:145-54. [Crossref] [PubMed]
- Pinto NP, Rhinesmith EW, Kim TY, et al. Long-Term Function after Pediatric Critical Illness: Results from the Survivor Outcomes Study*. Pediatr Crit Care Med 2017;18:e122-30. [Crossref] [PubMed]
- Pascucci RC. Prolonged weakness after extended mechanical ventilation in a child. Crit Care Med 1990;18:1181-2. [Crossref] [PubMed]
- van Mourik IDM, Beath SV, Brook GA, et al. Long-Term Nutritional and Neurodevelopmental Outcome of Liver Transplantation in Infants Aged Less Than 12 Months. J Pediatr Gastroenterol Nutr 2000;30:269-75. [Crossref] [PubMed]
- Braunschweig CA, Sheean PM, Peterson SJ, et al. Exploitation of diagnostic computed tomography scans to assess the impact of nutrition support on body composition changes in respiratory failure patients. JPEN J Parenter Enteral Nutr 2014;38:880-5. [Crossref] [PubMed]
- Fetterplace K, Beach LJ, MacIsaac C, et al. Associations between nutritional energy delivery, bioimpedance spectroscopy and functional outcomes in survivors of critical illness. J Hum Nutr Diet 2019;32:702-12. [Crossref] [PubMed]
- Hermans G, Casaer MP, Clerckx B, et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med 2013;1:621-9. [Crossref] [PubMed]
- Casaer MP, Langouche L, Coudyzer W, et al. Impact of Early Parenteral Nutrition on Muscle and Adipose Tissue Compartments During Critical Illness*. Crit Care Med 2013;41:2298-309. [Crossref] [PubMed]
- Needham DM, Dinglas VD, Morris PE, et al. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med 2013;188:567-76. [Crossref] [PubMed]
- Allingstrup MJ, Kondrup J, Wiis J, et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med 2017;43:1637-47. [Crossref] [PubMed]
- Puthucheary ZA, Astin R, Mcphail MJW, et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax 2018;73:926-35. [Crossref] [PubMed]
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874-82. [Crossref] [PubMed]
- Choong K, Koo KKY, Clark H, et al. Early Mobilization in Critically Ill Children: A Survey of Canadian Practice. Crit Care Med 2013;41:1745-53. [Crossref] [PubMed]
- Cuello-Garcia CA, Mai SHC, Simpson R, et al. Early Mobilization in Critically Ill Children: A Systematic Review. J Pediatr 2018;203:25-33.e6. [Crossref] [PubMed]
- Kudchadkar SR, Nelliot A, Awojoodu R, et al. Physical Rehabilitation in Critically Ill Children: A Multicenter Point Prevalence Study in the United States. Crit Care Med 2020;48:634-44. [Crossref] [PubMed]
- Ong C, Lee JH, Leow MKS, et al. Functional Outcomes and Physical Impairments in Pediatric Critical Care Survivors: A Scoping Review*. Pediatr Crit Care Med 2016;17:e247-59. [Crossref] [PubMed]
- Choong K, Awladthani S, Khawaji A, et al. Early Exercise in Critically Ill Youth and Children, a Preliminary Evaluation: The wEECYCLE Pilot Trial*. Pediatr Crit Care Med 2017;18:e546-54. [Crossref] [PubMed]
- Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 2009;12:78-85. [Crossref] [PubMed]
- Heyland DK, Day A, Clarke GJ, et al. Nutrition and Exercise in Critical Illness Trial (NEXIS Trial): a protocol of a multicentred, randomised controlled trial of combined cycle ergometry and amino acid supplementation commenced early during critical illness. BMJ open 2019;9:e027893. [Crossref] [PubMed]
Cite this article as: Ong C, Lee JH, Puthucheary ZA. Narrative review of muscle weakness and wasting in pediatric critical illness. Pediatr Med 2021;4:13.

