Metabolic alterations in the critically ill child: a narrative review
Introduction
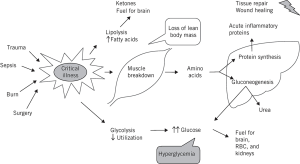
The acute metabolic response that follows an acute illness, trauma, and surgery is characterized by increased catabolism, release of increased amounts of glucose, amino acids (AA), and fatty acids from the body’s stores (1,2). Sir David P. Cuthbertson described the fundamental aspects of this metabolic response to injury more than half-a-century ago (3,4). This response varies depending of the nature and severity of the insult, as well as, factors related to the host (i.e., age, metabolic reserve capacity, and presence of chronic conditions) (5,6). This response includes changes in energy expenditure (EE), metabolic changes mediated by proinflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-12, IL-18, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ), hormonal responses with changes in levels of growth hormone (GH), thyroid-stimulating hormone (TSH), insulin growth factor binding proteins (IGFBP); and several metabolic reactions including increased gluconeogenesis, increased fatty acid and carbohydrate oxidation and increased loss of muscle mass (7-12) (Figure 1).
Methods
A comprehensive literature search was performed on March 31, 2020, for all papers published up to this date, using PubMed and Embase databases. The terms searched (MeSH heading) included: energy metabolism, critical illness, child, catabolism, stress, and inflammation. Conference abstracts, case reports, editorials, and non-English language articles were not included. Review papers were searched comprehensively to identify relevant articles. All articles relevant to the objectives of this review were approved by both authors.
Changes in EE
Energy metabolism is the most important function of the body and it regulates basal metabolism, growth, and physical activity; it is controlled at the cellular level by complex reactions of the neurohormonal system and regulates the utilization of substrate. The total objective of these controlling processes is to preserve energy stability and the central nervous system (CNS) acts an essential function in achieving this balance by triggering functions at the hormonal, neural, and metabolic level (13,14).
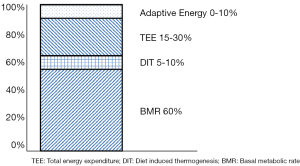
To be able to understand the concept of energy balance is essential to examine the elements of total energy expenditure (TEE) (Figure 2). TEE has four elements: basal metabolic rate (BMR), thermogenesis, physical activity, and adaptive energy. BMR accounts for 60–70%, thermogenesis for a 10% and physical activity represents 20-30% of TEE; adaptive energy is the energy expended to adapt to environmental conditions, particularly changes in ambient temperature; throughout circumstances of stress or injury these percentages varies depending on the degree of insult, substrate intake and amount of physical activity (15-19).
Energy needs are related to age and represents up to 3 to 4 times higher per body weight for infants compared to adults (20), and are also dependent on changes on metabolic rate and body nutrients reserve. In the presence of an insult the response will be proportional to the magnitude, nature, and duration of the injury (21). Increased levels of counter-regulatory hormones will result in opposition to the actions of insulin and GH. This increased resistance to the action of insulin and GH will result in a catabolic response with breakdown of glycogen, protein, and fat to provide enough substrate to support the metabolic response (22,23).
The main reason of the augmented energy dissipation in younger children is credited to the energy expense for growth. At 6 months of age the growth velocity is maximal representing up to 6–8% of energy utilized for growth, this process slows down at the age of 12 months once the BMR is 55 kcal/kg/day, when 2% of the EE is used for growth (16). During periods of acute stress, however, somatic growth is very difficult to achieve and cannot occur. Second, critically ill children are usually sedated or treated with muscle relaxants, therefore their activity level is reduced significantly lowering their energy needs. Third, the insensible losses are significantly reduced, particularly for patients on mechanical ventilatory support. Therefore, is very important to take into consideration these changes while implementing a nutrition support plan.
The traditional concept has been that acutely ill patients present a hypermetabolic condition known as “flow phase”, preceded by a phase of reduced EE aimed to preserve energy known as “ebb phase” (17,24-32). Many studies have reported measurements of EE by indirect calorimetry in children admitted to the pediatric intensive care unit (PICU) (15,17,24-46), the aggregate result of all these measurements yields an average metabolic index [measured energy expenditure (MEE)/predicted BMR] of 1.02±0.10 (SD), indicating an average metabolic condition. Of note are the studies that reported decreased MEE in postsurgical infants and neonates using indirect calorimetry and tracer methodology indicating a hypometabolic state (47-49), therefore, the importance of adjusting the caloric intake in this population of infants and neonates to avoid overfeeding.
In summary, the metabolic response is characterized by dysregulation of the energy metabolism, therefore, is important to understand and identify these changes during the acute phase of the injury in order to implement timely and appropriate interventions to support metabolically the acutely ill patient, while avoiding underfeeding and overfeeding (16,25-27,50).
Inflammatory response and cytokines
The metabolic response to tissue injury is initiated by activation of the cytokine cascade. Cytokines are a group of proteins (<40 kDa) created and distributed with the objective of cell signaling (51), and after binding to specific receptors, cytokines prompt initiation, multiplication, or relocation of target cells (52). Cytokines comprises a number of groups including: interleukins, chemokines, interferons, TNF, and growth factors. During sepsis interleukins are produced and include proteins released by leukocytes and endothelial cells and include pro- and anti-inflammatory types; the interleukins with pro-inflammatory activity [IL-1β, TNF-α, IL-18, IL-12, IL-17, INF-γ, and granulocyte-macrophage colony-stimulating factor (GM-CSF)] participate in cellular activation, tissue destruction, and necrosis; the interleukins with anti-inflammatory properties [IL-10, IL-13, IL-1ra, and transforming growth factor beta (TGF-β)] are responsible for dampening and reversing the inflammatory process (52-56).
The role of cytokines including TNF-α, IL-1β, IL-6, IL-8 as important mediators of infection and tissue injury, and INF-γ as metabolic mediator have been identified in many experimental studies. In subjects with sepsis who died, IL-1β levels were higher compared to subjects that survived, implying a link between elevated levels of IL-1β and outcome in patients with sepsis (57). Several studies have been performed to provide evidence for the role of endogenously produced TNF in the development of cachexia, muscle wasting, and decreased albumin synthesis (51,52,58). The most important supplier of IL-6 is macrophages at the tissue with elevated concentrations noted in several inflammatory conditions such as cardiovascular and autoimmune diseases, or neoplasia (52). Higher levels of IL-6 have been reported in pediatric patients with sepsis compared to subjects with systemic inflammation not associated with infection (59,60). Additionally, in children with sepsis a higher level of IL-6 is linked to more severity (61), making IL-6 a useful tool to predict outcome in sepsis. IL-8 functions include chemotaxis and neutrophil stimulation and several studies have found to be a good discriminator for survival. Wong et al. reported in two studies (62,63), higher levels of IL-8 in deceased pediatric patients with septic shock compared to subjects that survived, also found that a level of IL-8 ≤ to 220 pg/mL on admission to the intensive care unit was a good predictor of survival. Two reports in children with cancer found that a low level of IL-8 was a good predictor of a low risk of bacteremia (64), and that levels >300 pg/mL in conjunction with high C-reactive protein (CRP) in children >12 years of age were associated with worse outcome in this population (65).
CRP and procalcitonin
CRP was discovered in 1930 by Tillett and Francis (66) and was given this name because precipitated serum when pneumococcal cell wall C-polysaccharide was present. It is an acute-phase reactant made by hepatocytes when infection or tissue injury is present (67,68). The systemic response to tissue damage caused by an inflammatory or infectious trigger, results in the production of inflammatory cytokines such as, IL-1, IL-6, and TNF-α, these cytokines stimulate the synthesis of acute phase proteins in the liver, including CRP and procalcitonin (PCT) (69-71). Serum CRP concentrations multiplies every 8 hours and reach peak levels at 36–50 hours, with a half-life of 4–7 hours (68). The utility of CRP as a tool to make diagnosis has shown to have limitations given its sensitivity and specificity to distinguish among benign vs. severe bacterial infection or the presence of a non-bacterial infection process. A systematic review evaluating CRP to diagnose bacterial infection accurately in ambulatory pediatric patients with fever, reported a sensitivity of 77% and specificity of 79%; this low sensitivity value suggests that CRP cannot be used to exclude all bacterial infection (72). Additionally, it is useful to monitor response to treatment after a diagnosis of infection has been done, with serial persistent high CRP levels or higher levels after 48 hours indicate inadequate treatment (67). The more recent studies in the literature of the use of CRP as a diagnostic tool has focused on the comparison of its diagnostic accuracy with the use of PCT (68).
Procalcitonin is made by the thyroid to control serum calcium concentrations and constitutes a precursor of calcitonin and it is produced by the parafollicular cells (C cells) of the thyroid and by the neuroendocrine cells of the lung and the intestine, and thyroid C cells are the only ones that express the enzymes that produce the mature calcitonin (73). The thyroid gland produces PCT and this production occurs under normal conditions with low levels detected. Under an infection challenge the production of PCT by non-thyroidal tissue is increased significantly, suggesting that initial inflammatory stimulation from TNF-α, IL-1β and IL-6 is important (68). Multiple studies have evaluated the diagnostic use and advantage of using PCT to discriminate sepsis from systemic inflammatory response syndrome (SIRS) (74-78), and reported that children with established infection had elevated values of PCT compared to children without infection (SIRS only) (78), serum PCT concentration was significantly elevated in children with sepsis compared to children without infection with SIRS after cardiopulmonary bypass, (74), and in a cohort of children admitted to the PICU, PCT was better than CRP in discriminating subjects with SIRS and sepsis with PCT elevated concentrations associated with higher severity of illness (77). The level of evidence published to date in children with infection and sepsis, preclude the routine use of PCT as a biomarker in clinical practice, as a prognostic tool and risk stratification, or to help with the decision of antibiotic treatment duration (56).
Hormonal response
The stress responses to injury, trauma or sepsis are mediated by a number of different hormones, protein messengers, and the development of a complex system of neural injury-induced stimuli that triggers the CNS, resulting in alterations at the hypothalamic-anterior pituitary axes, these include the adrenal gland (increased cortisol secretion), the somatotrophic (increased GH secretion), the thyrotrophic [decreased triiodothyronine (T3) and increased reverse T3 (rT3) secretion], and the gonado-/lactotrophic (decreased testosterone, increased prolactin) axes (79,80). In addition, the CNS also acts through the peripheral sympathetic nervous system to increase catecholamine secretion.
After an insult, a condition of increased resistance to the actions of the GH at the peripheral tissues is developed (9,81), this response is in part a result of the secreted cytokines. The increase in circulating amounts of GH (82) is heralded by a reduction in concentrations of GH-binding protein, indicating a reduction on the expression of the GH receptor at the level of peripheral tissues (83,84). The reduction in negative feedback inhibition explains the ample availability of GH during the initial stages of the stress response; this answer of the GH axis is instrumental in the fight for existence, where indirect insulin-like growth factor-1 (IGF-1) mediated somatotrophic effects of GH are attenuated, resulting in increased levels in the circulation of glucose and fatty acids (80). The decreased level of somatotropism, because of a lack of pulsatile GH secretion might add to the etiology of the wasting syndrome that distinguishes by a protracted course of a severe condition (79,80,85).
Shortly after the onset of severe stress there is a rapid decline of circulating levels of T3 with a concomitant increase in rT3 as a result of disrupted peripheral conversion of T4 (86,87). The instant reduction in levels of T3 might be seen as an answer to preserve energy while the substrate intake is markedly reduced (80). The persistence of low T3 after normalization of TSH levels is known as the low euthyroid syndrome. The reduction in T3 levels throughout the initial period following the insult is a reflection of the severity of the disease process (88) and this has been shown in clinical studies where a low T3 is associated with increased mortality (79,89,90).
Cortisol levels increase during the acute phase of the response to injury as a result of the increased release of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), this is explained either by a direct mechanism or inhibition of the negative feedback by cortisol (91,92). The majority of the suppressive effects of cortisol on immune and inflammatory reactions appear to be a consequence of the modulation of production or activity of cytokines (i.e., IL-1, IL-2, IL-3, IL-6, interferon-γ, TNF-α), chemokines, eicosanoids, complement activation, and other inflammatory mediators (i.e., bradykinin, histamine, macrophage migration inhibitory factor) (93). The most necessary and dynamic hypercortisolism induced by stress in critically ill patients results in energy provision by shifting carbohydrate, fat, and protein metabolism, suppress inflammation, and boost hemodynamics by augmented sensitization of the vasopressor response to catecholamines (80,93,94). Plausible disadvantages of prolonged hypercortisolism include impaired wound healing and myopathy, complications often seen during lengthy course of critical illness (79,80,90,94).
Carbohydrate and lipid metabolism
During conditions of stress, hyperglycemia is a consequence of a mixture of enhanced gluconeogenesis and enhanced insulin resistance resulting in decreased glucose uptake by the cells (95) (Figure 1). These two mechanisms are potentially mediated by increases in counter regulatory hormones and proinflammatory cytokines and potentially these cytokines prevent insulin to be secreted by the pancreas via activation of α adrenergic receptors (96-98). Increased concentrations of counterregulatory hormones and proinflammatory cytokines participate in the regulation of glycogenolysis and gluconeogenesis with resultant hyperglycemia (95), and glycogen stores are rapidly depleted with glycogenolysis resulting in limited glucose production (96). The elevated concentrations of catecholamines during this acute response to injury results in elevated glucagon levels with gluconeogenesis being maintained despite elevated levels of insulin (99). Other hormonal changes including increased GH and decreased IGF-1 concentrations enable the destruction of muscle releasing alanine to enhance gluconeogenesis (100). Acute injury is distinguished by insulin resistance either central or peripheral (101), and insulin resistance at the hepatic level being central and mediated by glucagon, epinephrine, and cortisol (99). The Insulin resistance at the muscle and fat tissue is classified as peripheral and is explained by changes in the insulin-signaling pathway regulated by inflammatory cytokines and counter regulatory hormones (101). This peripheral insulin resistance might continue for a protracted time after recovery from an acute injury, as described in pediatric patients (102). Several studies have reported defects in beta-cell function of the pancreas with reduction in its ability to produce insulin in critically ill children (103,104).
The fat tissue represents the main supplier of fuel and it is deposited as triacylglycerides and the breakdown or lipolysis produces non-esterified free fatty acids (FFA) (105). Lipolysis at the extracellular and intracellular space is regulated by lipoprotein and hormone-sensitive lipase (HSL). During conditions of inflammation there is a significant increase in serum levels of triacylglycerides and FFA and decrease in high-density lipoprotein concentration (106). Additionally, increased levels of cytokines and catecholamines induce blockage of lipoprotein lipase and reduced extracellular lipolysis, simultaneously, upregulation of HSL results in lipolysis at the fat tissue (105). An increased triacylglycerides synthesis results in reduced clearance of triglycerides, resulting in hepatic steatosis (107), and storage of triacylglycerides and FFA at the muscle (108,109), heart (110), and kidney (111), this is the result of a disrupted uptake FFA and its oxidation. No reported definite evidence that an alteration of the FFA oxidation is present at the mitochondrial and peroxisomal level in the acutely ill patient, but in subjects with type 2 diabetes and metabolic syndrome, these alterations modify the insulin signaling and enhances insulin resistance (112,113). Hypertriglyceridemia is an important concern because of the altered endothelial function, lipotoxicity, and increased inflammation and often lipid infusion during acute injury conditions worsens hypertriglyceridemia (105).
AA and protein and metabolism in the critically ill
The complex interrelation of protein metabolism and metabolic partitioning during critically illness across the human lifespan emphasizes the need to individualize protein support therapy towards achievement of proteostasis (protein metabolic homeostasis) and organ support rather than simply balancing nitrogen expenditure (114-116). To sustain tissue integrity and organ function in healthy conditions, body protein is continuously degraded and resynthesized in all tissues and cells, a process known as protein turnover. Tissue proteins in different organs are constituted by AA, which normally can be either incorporated into tissue protein or undergo oxidation for energy production when energy intake is inadequate to satisfy the metabolic demands. Normally, tissue protein breakdown releases AA to the peripheral circulation, and those circulating AA may be reutilized for accretion of tissue protein or may perform intracellular or physiologic functions. Adult individuals are less efficient than children and neonates to convert dietary protein into net accretion and maintenance of body protein, but youngsters also require additional protein per unit of mass to include fractional needs required to sustain growth in their maintenance protein and AA requirements (115).
Skeletal muscle mass accounts for a major component of the lean body mass (LBM) as the largest protein reserve in the body. During illness, muscle and LBM correlates with severity of illness, systemic inflammation, impairment of the respiratory function and clinical outcomes in both pediatric and adult patients (117-119). Other components of the body protein reserve include circulating proteins, such as visceral proteins, acute phase reactants, hemoglobin, leucocytes, and immunoglobulins. In normal conditions, the balance between protein and AA intake, protein turnover, and nitrogen loss is aimed to maintain LBM, sustain protein compartments and homeostasis, and, in the case of children and neonates, also for lean mass growth (120). In critically ill states, pre-albumin and retinol-binding protein are more accurate to evaluate the response of the de novo plasma protein pool to dietary protein intake because of their shorter half-life, when compared to circulating proteins with a longer half-life, such as albumin (121,122).
For infants and pediatric patients, rapid growth occurs because of efficient protein accretion of skeletal muscle mass, mediated in large part by very high protein synthesis rates in skeletal muscle and extreme sensitivity to anabolic stimulation triggered by post prandial elevation of circulating insulin and AA. Such robust post prandial response to insulin and AA stimulation in the young does not affect muscle protein degradation and it declines as the young individual becomes an adult (123-126). Therefore, normal infants and children have a more efficient use of dietary protein and AA released from endogenous proteins breakdown to conserve and grow LBM.
Critical illness induces loss and catabolism of body protein by the presence of starvation, immobility, stress, and inflammation. With current advances in intensive care and life sustaining support with extracorporeal therapies, dialysis, mechanical ventilation, medications (such as steroids, sedatives, and immunosuppressors) and the presence of organ dysfunction can cause prolongation of the inflammatory and catabolic state and add to the promotion of nitrogen loss. Such prolongation of the catabolic state creates a chronic cumulative nitrogen deficit (127).
Nitrogen shuttle and metabolic partitioning
During critical illness, in contrast to normal states, injury and inflammation induce protein breakdown release AA and nitrogen to the systemic circulation to provide substrate for whole body protein metabolism (128-130) (Figure 3A,B). Such metabolic response is not reversed by provision of exogenous protein as is innately driven and regulated by stress hormones, neural mediators, and cytokines. Systemic inflammation enhances protein synthesis in the liver and immune cells displaying as increased whole body protein synthesis rates. Circulating plasma AA released from body protein are preferentially used for gluconeogenesis, oxidation to produce energy, as substrate for immune cells and enterocyte metabolism, and to supply nitrogen to the liver for synthesis of acute phase reactants. Therefore, circulating plasma AA concentrations are extracted from the systemic circulation and thus achieve lower levels in patients with critical illness when compared to healthy subjects (128,130). Intestinal epithelial breakdown and a decrease in visceral protein synthesis (i.e., albumin, and pre-albumin) ensues when protein or AA are not provided in the enteral lumen for its absorption and release to the splanchnic bed (131,132). In healthy conditions, portal rather than arterial AA is preferentially used for hepatic protein synthesis of visceral protein after enteral feeding (132). Protein metabolic partitioning occurs based on specific organ needs, as different organ systems may require and uptake specific AA or when a particular AA may serve as a precursor or as a physiologic signal during critical illness (115). Thirty to fifty percent of essential AA in the diet may be catabolized by the small intestine in first-pass metabolism for enteral utilization by the enterocyte and splanchnic extraction (133,134).
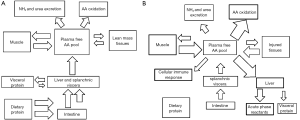
As opposed to the increase in whole body protein synthesis during systemic inflammatory states, in skeletal muscle protein synthesis decreases and protein degradation increases, to decrease uptake and utilization of AA by muscle tissue and to release and shuttle AA and nitrogen to the immune cells and visceral tissues (135,136). This preference on protein degradation over protein synthesis in skeletal muscle leads to muscle atrophy and loss of LBM, and it is also associated with growth failure in children (137,138). In this regard, critically ill children have a higher protein turnover than adults, due to relatively amplified baseline higher whole-body protein synthesis and breakdown, limiting loss of LBM by their protective robust baseline anabolic rates (135,139). Critically ill adults can achieve maximal rate of protein loss in the first 10 days, and loose more than 14% of total body protein over 3 weeks (140,141).
From studying fast or slow proteins in animal models and humans, it appears that is the rapid increase and variation in the plasma AA concentrations what leads to protein synthesis in muscle, not the absolute AA concentrations. In neonatal animal studies, intermittent boluses of protein have improved feeding efficiency, by inducing a greater stimulatory effect on skeletal muscle protein synthesis than continuous enteral feedings (126,142,143).
Intracellular protein turnover in critical illness
In skeletal muscle and in most organs, cellular protein mass or function are maintained by regulation of the protein synthesis and degradation balance (Figure 4). Protein synthesis in all organs occurs by triggering of a signaling pathway that stimulates translation of mRNA into protein and it can be regulated differently in different organs during critical illness. In this regard, systemic inflammation increases hepatic protein synthesis by activating the translational machinery while simultaneously impairing the efficiency of translation of mRNA into protein in muscle (144,145). Protein degradation in skeletal muscle is regulated by molecular signals involved in translation (146). Protein kinase B (PKB, also known as Akt) an insulin signaling protein, appears to link translation and protein degradation signal activation. Translation comprises activation of the mammalian target of rapamycin (mTOR) through PKB and intracellular AA. Intracellular AA also activates translation initiation via stimulation of mRNA binding to the 43S ribosomal complex; and through eIF2B, which stimulates the binding of the initiator methionyl-tRNA (met-tRNAi) to form the 43S pre-initiation complex; and dephosphorylation of the eukaryotic elongation factor 2 (eEF2) for peptide chain elongation (144,147). Systemic circulating AA require active transmembrane transport to become intracellular. PKB activation also inhibits Caspase-3 activity and restrains activation of the Fox0 group of proteins. Proteases such as caspase-3 facilitate intact muscle fiber decomposition to release monomeric contractile proteins, such as actin and myosin, for further disintegration into AA by the ubiquitin-proteasome system (148). PKB and mTOR inhibition increase E3 ubiquitin ligase expression of muscle atrophy F-box (MAFbx, atrogin1) and muscle RING finger 1 (MuRF1), which have been associated with activation of the ubiquitin-proteosomal system (149,150). High protein synthesis rates in young animals are due to an enhanced translational process that declines as the animal matures (125,151). In contrast, animal studies suggest that the more intense activation of degradation signaling at baseline in skeletal muscle of young animals cannot be enhanced by inflammation, and that catabolic signal activation in skeletal manifests its severity as maturation advances (150). Autophagy appears to be an innate process that is activated by inflammation and antagonized by the presence of intracellular AA, which can antagonize autophagy signal activation (152). Moreover, protein synthesis and degradation in skeletal muscle can be regulated by the presence or absence of fiber stretch, and immobility leads to enhanced catabolic processes and decreased protein synthesis (153).
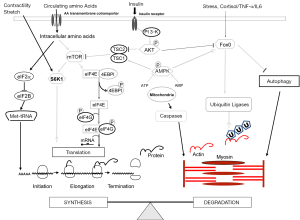
Alteration in energy metabolism during systemic inflammatory states leads to decreased translation and enhanced degradation signal activation in skeletal muscle. Inflammation may cause mitochondrial dysfunction and energy failure causing enhanced catabolic signals and decreased protein synthesis. 5’-AMP-activated protein kinase (AMPK), an intracellular energy sensor, is activated in the presence of energy starvation, inhibits mTOR and protein synthesis signal activation and activates the ubiquitin-proteosomal system (149,150,154). In neonatal animals, insulin has shown to antagonize AMPK activation and thus, appears to stimulate protein synthesis and decrease muscle protein degradation signal activation in skeletal muscle during inflammation, suggesting that insulin resistance plays a role in skeletal muscle catabolism during critical illness (136,154).
Protein catabolism and anabolic resistance
Critical illness is a rapidly changing physiologic state, in which protein requirements, utilization and balance is evolving in accordance to the progression of the acute physiologic alterations. Critical illness may induce a catabolic response and a loss of LBM that may be unresponsive to exogenous nutrient support, in contrast to simple starvation (137). During critical illness, the effects of the autonomic stress response, insulin resistance, cortisol, cytokines, and the dysfunction of anabolic hormones may decrease the expected response to adequate protein provision. Both injury and inflammation lessen the response to anabolic hormones and nutrients that enhance protein deposition in skeletal muscle and maintenance of the LBM (141,144).
To preserve LBM, circulating insulin and its response are crucial for skeletal muscle protein deposition, as they stimulate protein synthesis, inhibit muscle protein degradation, and improves energy homeostasis in skeletal muscle (142,154). In this regard, insulin continues to stimulate skeletal muscle protein synthesis and inhibits muscle protein degradation during critical illness but does not attenuate whole body proteolysis when provided at higher than physiological concentrations (155-158), possibly due to the antagonism of circulating cytokines (105,159). As we explained previously, assessment of the response of protein metabolism to insulin at the whole-body level may not reflect the favorable effects of insulin in skeletal muscle during critical illness, since insulin does not affect the elevated protein synthesis rates in liver during systemic inflammation (160). Thus, due to such metabolic partitioning during critical illness, the advantageous effects of insulin on whole body protein metabolism are permissive for protein synthesis and suppressive for protein breakdown only if adequate AA are provided (105,136,157). In addition, insulin has been reported to have intrinsic anti-inflammatory properties and positive effects on reestablishing glucose and energy homeostasis and stimulation of protein anabolism in skeletal muscle (157,161-163).
In pediatric critical illness other important mediators of the stress response such as corticosteroids cause insulin resistance, hyperglycemia, net release of glutamine from muscle, and decrease in translation initiation and enhancement of protein degradation in muscle (130,164). While the epinephrine and norepinephrine are usually associated with catabolic processes on energy metabolic rate, they may have an anabolic effect on skeletal muscle protein metabolism (165). Critical illness is associated with transitory reduced levels of IGF-1, acquired GH resistance, and a decreased anabolic response to GH (141).
Branched-chain AA (leucine, isoleucine, valine), threonine, and lysine supply close to the 75% of the body’s nitrogen requirement (166). Even though certain AA may directly exert physiologic or cellular effects, AA imbalances may also be negative for metabolic homeostasis, and during critical illness they may become conditionally essential. That is because all 20 protein AA and their metabolites are required for normal cell physiology and function, and their single deficiency or oversupply may blunt their intrinsic beneficial effects (167). AA are intrinsically anabolic and can stimulate a marked rise in muscle protein synthesis independent of insulin stimulation. AA requirements are also influenced by age because of increased requirements in the presence of active growth in the young individual (157). In critical illness, Alanine, Glutamine, Glycine, and Aspartic acid can act as gluconeogenic substrates, shuttling nitrogen from peripheral skeletal muscle to the circulating AA pool. Glutamine is a major constituent in muscle protein, shuttling about one-third of all AA nitrogen and serves as fuel for enterocytes and cellular immune response (168). Arginine, and its precursor citrulline, are precursors of nitric oxide, creatine, agmatine and other polyamines, and modulates protein anabolism (133,167,169). Parenteral BCAA have been used to improved outcomes in critical illness without success (128). Leucine, and its metabolite beta-hydroxy-beta-methylbutyrate, have a direct anabolic effect in skeletal muscle, and have been used to stimulate nitrogen maintenance (152,170,171).
The hypercatabolic state of injury or sepsis has been characterized a marked negative nitrogen balance (25,27,50,136,172). Nitrogen excretion is linked to the metabolic expenditure because it is affected by severity of illness. Whole body nitrogen utilization is affected by energetic deficits, and protein can also be oxidized for energy in catabolic states (25,27). During intensive care support during critical illness, nitrogen can be lost in urine, stool, skin, and in extracorporeal elements such as dialysate, extracorporeal circuits and thoracic or abdominal drainage (173-176). Therefore, even when provided with the appropriate estimated requirements, the critically ill may lose more protein than that able to assimilate (173). Although aiming for a positive protein balance has been used as a surrogate measure of LBM preservation, it does not assess protein or AA utilization, quality of intake or protein reserves or metabolic partitioning. Moreover, sufficient amounts of energy are needed to efficiently utilize the supplemented protein. When protein and energy are supplied during critical illness, whole body protein synthesis rates are increased without affecting protein breakdown. Therefore, improvement in protein balance at the expense of higher protein synthesis may occur despite resultant ongoing losses of body protein and attaining protein balance may not prevent loss of LBM or skeletal muscle mass (27,173,177).
Even when faced with a critical illness, infants and children contrast from adults in their requirement for a continuous supply of substrate and energy to maintain growth and their protein needs. Acceptable quantities of energy are needed to efficiently use the supplemented protein, since whole body nitrogen utilization is affected by energetic deficits, and protein is catabolized to loss and oxidized for energy in catabolic conditions (25). It is recommended to adjust the normal caloric partitioning (50–60% of calories from carbohydrates, 25–35% from protein, and 10–25% from fat) to adjust to the increased protein needs to prevent AA to be used for energy production during critical illness. The calculation of calorie-to-nitrogen ratio, whether total or non-protein calories, supports the concept of providing adequate caloric intake when high protein is provided (178). Traditionally, and based on expert opinion, the recommended calorie-to-nitrogen ratio requirement has been suggested around 130–150 kcal/gram of nitrogen (1 gram of protein =6.25 grams of nitrogen) during critical illness in adults. In contrast, an energy to nitrogen (E/N) expenditure ratio of 382:1 kcal/gram of nitrogen has been described in healthy active young men and was proposed to help design the adequate caloric partitioning for enteral nutrition or parenteral nutrition support (173). In that report, the E/N ratio decreases continuously with increasing protein loss and is not a constant value (173). This evidence indicates the need for studies that specifically match intake to expenditures in critically ill individuals across the lifespan to encompass for the large variations in EE, protein loss, and E/N ratios in diverse patient populations.
Proteostasis in the critically ill child implies understanding protein metabolism and adaptation to stress (115). Studies on protein metabolism discovered that humans adapt to prolonged low protein intake and maintain of health and LBM (179) by means of metabolic adaptation and plasticity, metabolomics and epigenetics (167,180). During conditions of protein starvation, cells respond to the stress of AA deprivation through sensing the AA deficiency, leading to modulation of global protein synthesis to save EE through translation reprogramming to maintain metabolic homeostasis (180). Adequate understanding of energy and macronutrient sustenance to the metabolic adaptation to prolonged survival in intensive care when supporting critical illness will allow improved survival and recovery, and in children, restoration of growth potential.
Acknowledgments
Funding: Financial and material support from the Section of Critical Care and the Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyvonne Tume, Frederic Valla and Sascha Verbruggen) for the series “Nutrition in the Critically Ill Child” published in Pediatric Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-64). The series “Nutrition in the Critically Ill Child” was commissioned by the editorial office without any funding or sponsorship. JACB reports personal fees from Nestle USA, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cogo PE, Carnielli VP, Rosso F, et al. Protein turnover, lipolysis, and endogenous hormonal secretion in critically ill children. Crit Care Med 2002;30:65-70. [Crossref] [PubMed]
- Shaw JH, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg 1987;205:288-94. [Crossref] [PubMed]
- Cuthbertson DP. Further observations on the disturbance of metabolism caused by injury, with particular reference to the dietary requirements of fracture cases. 1936;23:505-20.
- Cuthbertson DP. Second annual Jonathan E. Rhoads Lecture. The metabolic response to injury and its nutritional implications: retrospect and prospect. JPEN J Parenter Enteral Nutr 1979;3:108-29. [Crossref] [PubMed]
- Hasselgren PO. Catabolic response to stress and injury: implications for regulation. World J Surg 2000;24:1452-9. [Crossref] [PubMed]
- Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg 2008;248:387-401. [PubMed]
- Baumann H, Gauldie J. The acute phase response. Immunol Today 1994;15:74-80. [Crossref] [PubMed]
- Dahn MS, Jacobs LA, Smith S, et al. The relationship of insulin production to glucose metabolism in severe sepsis. Arch Surg 1985;120:166-72. [Crossref] [PubMed]
- Ross R, Miell J, Freeman E, et al. Critically ill patients have high basal growth hormone levels with attenuated oscillatory activity associated with low levels of insulin-like growth factor-I. Clin Endocrinol (Oxf) 1991;35:47-54. [Crossref] [PubMed]
- Shaw JH, Wolfe RR. Determinations of glucose turnover and oxidation in normal volunteers and septic patients using stable and radio-isotopes: the response to glucose infusion and total parenteral feeding. Aust N Z J Surg 1986;56:785-91. [Crossref] [PubMed]
- Wilmore DW. Catabolic illness. Strategies for enhancing recovery. N Engl J Med 1991;325:695-702. [Crossref] [PubMed]
- Wolfe RR, Jahoor F, Herndon DN, et al. Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion. Surgery 1991;110:54-67. [PubMed]
- Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev 2006;86:651-67. [Crossref] [PubMed]
- Sisley S, Sandoval D. Hypothalamic control of energy and glucose metabolism. Rev Endocr Metab Disord 2011;12:219-33. [Crossref] [PubMed]
- Chwals WJ, Lally KP, Woolley MM, et al. Measured energy expenditure in critically ill infants and young children. J Surg Res 1988;44:467-72. [Crossref] [PubMed]
- Coss-Bu JA, Mehta NM. Energy Metabolism. In: Caballero B, Finglas P, Toldrá F. editors. The Encyclopedia of Food and Health. Oxford: Academic Press, 2016:503-10.
- Framson CM, LeLeiko NS, Dallal GE, et al. Energy expenditure in critically ill children. Pediatr Crit Care Med 2007;8:264-7. [Crossref] [PubMed]
- Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985;39:5-41. [PubMed]
- Talbot FB. Basal metabolism standards for children. Am J Dis Child 1938;55:455-9.
- Holliday MA. Metabolic rate and organ size during growth from infancy to maturity and during late gastation and early infancy. Pediatrics 1971;47:169. [Crossref] [PubMed]
- Chwals WJ. Overfeeding the critically ill child: fact or fantasy? New Horiz 1994;2:147-55. [PubMed]
- Bier DM. Growth hormone and insulin-like growth factor I: nutritional pathophysiology and therapeutic potential. Acta Paediatr Scand Suppl 1991;374:119-28. [Crossref] [PubMed]
- Chwals WJ, Bistrian BR. Role of exogenous growth hormone and insulin-like growth factor I in malnutrition and acute metabolic stress: a hypothesis. Crit Care Med 1991;19:1317-22. [Crossref] [PubMed]
- Briassoulis G, Venkataraman S, Thompson AE. Energy expenditure in critically ill children. Crit Care Med 2000;28:1166-72. [Crossref] [PubMed]
- Coss-Bu JA, Jefferson LS, Walding D, et al. Resting energy expenditure and nitrogen balance in critically ill pediatric patients on mechanical ventilation. Nutrition 1998;14:649-52. [Crossref] [PubMed]
- Coss-Bu JA, Jefferson LS, Walding D, et al. Resting energy expenditure in children in a pediatric intensive care unit: comparison of Harris-Benedict and Talbot predictions with indirect calorimetry values. Am J Clin Nutr 1998;67:74-80. [Crossref] [PubMed]
- Coss-Bu JA, Klish WJ, Walding D, et al. Energy metabolism, nitrogen balance, and substrate utilization in critically ill children. Am J Clin Nutr 2001;74:664-9. [Crossref] [PubMed]
- Havalad S, Quaid MA, Sapiega V. Energy expenditure in children with severe head injury: lack of agreement between measured and estimated energy expenditure. Nutr Clin Pract 2006;21:175-81. [Crossref] [PubMed]
- Kerklaan D, Hulst JM, Verhoeven JJ, et al. Use of Indirect Calorimetry to Detect Overfeeding in Critically Ill Children: Finding the Appropriate Definition. J Pediatr Gastroenterol Nutr 2016;63:445-450. [Crossref] [PubMed]
- Mehta NM, Bechard LJ, Leavitt K, et al. Cumulative energy imbalance in the pediatric intensive care unit: role of targeted indirect calorimetry. JPEN J Parenter Enteral Nutr 2009;33:336-44. [Crossref] [PubMed]
- Sy J, Gourishankar A, Gordon WE, et al. Bicarbonate kinetics and predicted energy expenditure in critically ill children. Am J Clin Nutr 2008;88:340-7. [Crossref] [PubMed]
- Vazquez Martinez JL, Martinez-Romillo PD, Diez Sebastian J, et al. Predicted versus measured energy expenditure by continuous, online indirect calorimetry in ventilated, critically ill children during the early postinjury period. Pediatr Crit Care Med 2004;5:19-27. [Crossref] [PubMed]
- Chwals WJ, Bistrian BR. Predicted energy expenditure in critically ill children: problems associated with increased variability. Crit Care Med 2000;28:2655-6. [Crossref] [PubMed]
- Chwals WJ, Lally KP, Woolley MM. Indirect calorimetry in mechanically ventilated infants and children: measurement accuracy with absence of audible airleak. Crit Care Med 1992;20:768-70. [Crossref] [PubMed]
- de Klerk G, Hop WC, de Hoog M, et al. Serial measurements of energy expenditure in critically ill children: useful in optimizing nutritional therapy? Intensive Care Med 2002;28:1781-5. [Crossref] [PubMed]
- Joosten KF, Verhoeven JJ, Hazelzet JA. Energy expenditure and substrate utilization in mechanically ventilated children. Nutrition 1999;15:444-8. [Crossref] [PubMed]
- López-Herce Cid J, Sanchez Sanchez C, Mencia Bartolome S, et al. Energy expenditure in critically ill children: correlation with clinical characteristics, caloric intake, and predictive equations. An Pediatr (Barc) 2007;66:229-39. [PubMed]
- Oosterveld MJ, Van Der Kuip M, De Meer K, et al. Energy expenditure and balance following pediatric intensive care unit admission: a longitudinal study of critically ill children. Pediatr Crit Care Med 2006;7:147-53. [Crossref] [PubMed]
- Selby AM, McCauley JC, Schell DN, et al. Indirect calorimetry in mechanically ventilated children: a new technique that overcomes the problem of endotracheal tube leak. Crit Care Med 1995;23:365-70. [Crossref] [PubMed]
- Taylor RM, Cheeseman P, Preedy V, et al. Can energy expenditure be predicted in critically ill children? Pediatr Crit Care Med 2003;4:176-180. [Crossref] [PubMed]
- van der Kuip M, de Meer K, Oosterveld MJ, et al. Simple and accurate assessment of energy expenditure in ventilated paediatric intensive care patients. Clinical nutrition 2004;23:657-63. [Crossref] [PubMed]
- van der Kuip M, de Meer K, Westerterp KR, et al. Physical activity as a determinant of total energy expenditure in critically ill children. Clinical nutrition 2007;26:744-51. [Crossref] [PubMed]
- Verhoeven JJ, Hazelzet JA, van der Voort E, et al. Comparison of measured and predicted energy expenditure in mechanically ventilated children. Intensive Care Med 1998;24:464-8. [Crossref] [PubMed]
- White MS, Shepherd RW, McEniery JA. Energy expenditure measurements in ventilated critically ill children: within- and between-day variability. JPEN J Parenter Enteral Nutr 1999;23:300-4. [Crossref] [PubMed]
- White MS, Shepherd RW, McEniery JA. Energy expenditure in 100 ventilated, critically ill children: improving the accuracy of predictive equations. Crit Care Med 2000;28:2307-12. [Crossref] [PubMed]
- Witte MK. Metabolic measurements during mechanical ventilation in the pediatric intensive care unit. Respir Care Clin N Am 1996;2:573-86. [PubMed]
- Alaedeen DI, Queen AL, Leung E, et al. C-Reactive protein-determined injury severity: length of stay predictor in surgical infants. J Pediatr Surg 2004;39:1832-4. [Crossref] [PubMed]
- Jaksic T, Shew SB, Keshen TH, et al. Do critically ill surgical neonates have increased energy expenditure? J Pediatr Surg 2001;36:63-7. [Crossref] [PubMed]
- Letton RW, Chwals WJ, Jamie A, et al. Early postoperative alterations in infant energy use increase the risk of overfeeding. J Pediatr Surg 1995;30:988-92; discussion 992-3. [Crossref] [PubMed]
- Coss-Bu JA, Hamilton-Reeves J, Patel JJ, et al. Protein Requirements of the Critically Ill Pediatric Patient. Nutr Clin Pract 2017;32:128S-141S. [Crossref] [PubMed]
- Dinarello CA. Historical insights into cytokines. Eur J Immunol 2007;37:S34-45. [Crossref] [PubMed]
- Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 2017;39:517-28. [Crossref] [PubMed]
- Behrens EM, Koretzky GA. Review. Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol 2017;69:1135-43. [Crossref] [PubMed]
- Canna SW, Behrens EM. Making sense of the cytokine storm: a conceptual framework for understanding, diagnosing, and treating hemophagocytic syndromes. Pediatr Clin North Am 2012;59:329-44. [Crossref] [PubMed]
- Hall MW, Greathouse KC, Thakkar RK, et al. Immunoparalysis in Pediatric Critical Care. Pediatr Clin North Am 2017;64:1089-1102. [Crossref] [PubMed]
- Lanziotti VS, Póvoa P, Soares M, et al. Use of biomarkers in pediatric sepsis: literature review. Rev Bras Ter Intensiva 2016;28:472-82. [Crossref] [PubMed]
- Mera S, Tatulescu D, Cismaru C, et al. Multiplex cytokine profiling in patients with sepsis. APMIS 2011;119:155-63. [Crossref] [PubMed]
- Chang HR, Bistrian B. The role of cytokines in the catabolic consequences of infection and injury. JPEN J Parenter Enteral Nutr 1998;22:156-66. [Crossref] [PubMed]
- Huang SY, Tang RB, Chen SJ, et al. Serum interleukin-6 level as a diagnostic test in children with sepsis. J Chin Med Assoc 2003;66:523-7. [PubMed]
- Zurek J, Vavrina M. Procalcitonin biomarker kinetics to predict multiorgan dysfunction syndrome in children with sepsis and systemic inflammatory response syndrome. Iran J Pediatr 2015;25:e324. [PubMed]
- Pavare J, Grope I, Kalnins I, et al. High-mobility group box-1 protein, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in children with community acquired infections and bacteraemia: a prospective study. BMC Infect Dis 2010;10:28. [Crossref] [PubMed]
- Wong HR. Pediatric septic shock treatment: new clues from genomic profiling. Pharmacogenomics 2007;8:1287-90. [Crossref] [PubMed]
- Wong HR, Cvijanovich N, Wheeler DS, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med 2008;178:276-82. [Crossref] [PubMed]
- Cost CR, Stegner MM, Leonard D, et al. IL-8 predicts pediatric oncology patients with febrile neutropenia at low risk for bacteremia. J Pediatr Hematol Oncol 2013;35:206-11. [Crossref] [PubMed]
- Santolaya ME, Alvarez AM, Aviles CL, et al. Prospective validation of a risk prediction model for severe sepsis in children with cancer and high-risk febrile neutropenia. Pediatr Infect Dis J 2013;32:1318-23. [Crossref] [PubMed]
- Tillett WS, Francis T. Serological reactions in penumonia with a non-protein somatic fraction of penumococcus. J Exp Med 1930;52:561-71. [Crossref] [PubMed]
- McWilliam S, Riordan A. How to use: C-reactive protein. Arch Dis Child Educ Pract Ed 2010;95:55-8. [Crossref] [PubMed]
- Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther 2011;9:71-9. [Crossref] [PubMed]
- Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med 1992;37:313-36. [PubMed]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448-54. [Crossref] [PubMed]
- Kushner I. C-reactive protein and the acute-phase response. Hosp Pract (Off Ed) 1990;25:13-16, 21-8. [PubMed]
- Sanders S, Barnett A, Correa-Velez I, et al. Systematic review of the diagnostic accuracy of C-reactive protein to detect bacterial infection in nonhospitalized infants and children with fever. J Pediatr 2008;153:570-4. [Crossref] [PubMed]
- Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med 2008;36:941-52. [Crossref] [PubMed]
- Arkader R, Troster EJ, Lopes MR, et al. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch Dis Child 2006;91:117-20. [Crossref] [PubMed]
- Casado-Flores J, Blanco-Quiros A, Asensio J, et al. Serum procalcitonin in children with suspected sepsis: a comparison with C-reactive protein and neutrophil count. Pediatr Crit Care Med 2003;4:190-5. [Crossref] [PubMed]
- Fioretto JR, Martin JG, Kurokawa CS, et al. Comparison between procalcitonin and C-reactive protein for early diagnosis of children with sepsis or septic shock. Inflamm Res 2010;59:581-6. [Crossref] [PubMed]
- Rey C, Los Arcos M, Concha A, et al. Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med 2007;33:477-84. [Crossref] [PubMed]
- Simon L, Saint-Louis P, Amre DK, et al. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med 2008;9:407-13. [Crossref] [PubMed]
- Langouche L, Van den Berghe G. The dynamic neuroendocrine response to critical illness. Endocrinol Metab Clin North Am 2006;35:777-791. ix. [Crossref] [PubMed]
- Vanhorebeek I, Van den Berghe G. The neuroendocrine response to critical illness is a dynamic process. Crit Care Clin 2006;22:1-15. v. [Crossref] [PubMed]
- Baxter RC, Hawker FH, To C, et al. Thirty-day monitoring of insulin-like growth factors and their binding proteins in intensive care unit patients. Growth Horm IGF Res 1998;8:455-63. [Crossref] [PubMed]
- Voerman HJ, Strack van Schijndel RJ, de Boer H, et al. Growth hormone: secretion and administration in catabolic adult patients, with emphasis on the critically ill patient. Neth J Med 1992;41:229-44. [PubMed]
- Defalque D, Brandt N, Ketelslegers JM, et al. GH insensitivity induced by endotoxin injection is associated with decreased liver GH receptors. Am J Physiol 1999;276:E565-72. [PubMed]
- Hermansson M, Wickelgren RB, Hammarqvist F, et al. Measurement of human growth hormone receptor messenger ribonucleic acid by a quantitative polymerase chain reaction-based assay: demonstration of reduced expression after elective surgery. J Clin Endocrinol Metab 1997;82:421-8. [PubMed]
- Van den Berghe G, Wouters P, Weekers F, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 1999;84:1311-23. [PubMed]
- Chopra IJ, Huang TS, Beredo A, et al. Evidence for an inhibitor of extrathyroidal conversion of thyroxine to 3,5,3'-triiodothyronine in sera of patients with nonthyroidal illnesses. J Clin Endocrinol Metab 1985;60:666-72. [Crossref] [PubMed]
- Michalaki M, Vagenakis AG, Makri M, et al. Dissociation of the early decline in serum T concentration and serum IL-6 rise and TNFalpha in nonthyroidal illness syndrome induced by abdominal surgery. J Clin Endocrinol Metab 2001;86:4198-205. [PubMed]
- Rothwell PM, Lawler PG. Prediction of outcome in intensive care patients using endocrine parameters. Crit Care Med 1995;23:78-83. [Crossref] [PubMed]
- McIver B, Gorman CA. Euthyroid sick syndrome: an overview. Thyroid 1997;7:125-32. [Crossref] [PubMed]
- Van den Berghe G. Novel insights into the neuroendocrinology of critical illness. Eur J Endocrinol 2000;143:1-13. [Crossref] [PubMed]
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med 2003;348:727-34. [Crossref] [PubMed]
- Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature 1983;305:325-7. [Crossref] [PubMed]
- Marik PE, Zaloga GP. Adrenal insufficiency in the critically ill: a new look at an old problem. Chest 2002;122:1784-96. [Crossref] [PubMed]
- Van den Berghe G, de Zegher F, Bouillon R. Clinical review 95: Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab 1998;83:1827-34. [PubMed]
- Mechanick JI. Metabolic mechanisms of stress hyperglycemia. JPEN J Parenter Enteral Nutr 2006;30:157-63. [Crossref] [PubMed]
- Dufour S, Lebon V, Shulman GI, et al. Regulation of net hepatic glycogenolysis and gluconeogenesis by epinephrine in humans. Am J Physiol Endocrinol Metab 2009;297:E231-5. [Crossref] [PubMed]
- Kulp GA, Herndon DN, Lee JO, et al. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock 2010;33:369-74. [Crossref] [PubMed]
- Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab 2001;15:533-51. [Crossref] [PubMed]
- Shamoon H, Hendler R, Sherwin RS. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab 1981;52:1235-41. [Crossref] [PubMed]
- Gardelis JG, Hatzis TD, Stamogiannou LN, et al. Activity of the growth hormone/insulin-like growth factor-I axis in critically ill children. J Pediatr Endocrinol Metab 2005;18:363-72. [Crossref] [PubMed]
- Srinivasan V. Stress hyperglycemia in pediatric critical illness: the intensive care unit adds to the stress! J Diabetes Sci Technol 2012;6:37-47. [Crossref] [PubMed]
- Gauglitz GG, Herndon DN, Kulp GA, et al. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab 2009;94:1656-64. [Crossref] [PubMed]
- Preissig CM, Rigby MR. Hyperglycaemia results from beta-cell dysfunction in critically ill children with respiratory and cardiovascular failure: a prospective observational study. Crit Care 2009;13:R27. [Crossref] [PubMed]
- van Waardenburg DA, Jansen TC, Vos GD, et al. Hyperglycemia in children with meningococcal sepsis and septic shock: the relation between plasma levels of insulin and inflammatory mediators. J Clin Endocrinol Metab 2006;91:3916-21. [Crossref] [PubMed]
- Dhar A, Castillo L. Insulin resistance in critical illness. Curr Opin Pediatr 2011;23:269-74. [Crossref] [PubMed]
- Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 2004;45:1169-96. [Crossref] [PubMed]
- Wu A, Hinds CJ, Thiemermann C. High-density lipoproteins in sepsis and septic shock: metabolism, actions, and therapeutic applications. Shock 2004;21:210-21. [Crossref] [PubMed]
- Cree MG, Zwetsloot JJ, Herndon DN, et al. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg 2007;245:214-21. [Crossref] [PubMed]
- Feingold KR, Moser A, Patzek SM, et al. Infection decreases fatty acid oxidation and nuclear hormone receptors in the diaphragm. J Lipid Res 2009;50:2055-63. [Crossref] [PubMed]
- Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 2004;18:1692-700. [Crossref] [PubMed]
- Feingold KR, Wang Y, Moser A, et al. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J Lipid Res 2008;49:2179-87. [Crossref] [PubMed]
- Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med 2010;16:400-2. [Crossref] [PubMed]
- Zhang D, Liu ZX, Choi CS, et al. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci U S A 2007;104:17075-80. [Crossref] [PubMed]
- Hoffer LJ. Protein and energy provision in critical illness. Am J Clin Nutr 2003;78:906-11. [Crossref] [PubMed]
- Orellana RA, Coss-Bu JA. Energy and macronutrient requirements in the critically ill child. In: Praveen G, Nilesh M. editors. Pediatric Critical Care Nutrition. 1st edition. Nueva York, NY: McGraw-Hill Education, 2015:33-58.
- Singer P. Toward protein-energy goal-oriented therapy? Crit Care 2009;13:188. [Crossref] [PubMed]
- Engelen MP, Schroder R, Van der Hoorn K, et al. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clin Nutr 2012;31:927-33. [Crossref] [PubMed]
- Ionescu AA, Evans WD, Pettit RJ, et al. Hidden depletion of fat-free mass and bone mineral density in adults with cystic fibrosis. Chest 2003;124:2220-8. [Crossref] [PubMed]
- Thomson MA, Quirk P, Swanson CE, et al. Nutritional growth retardation is associated with defective lung growth in cystic fibrosis: a preventable determinant of progressive pulmonary dysfunction. Nutrition 1995;11:350-4. [PubMed]
- Butte NF, Hopkinson JM, Wong WW, et al. Body composition during the first 2 years of life: an updated reference. Pediatr Res 2000;47:578-85. [Crossref] [PubMed]
- Hulst JM, van Goudoever JB, Zimmermann LJ, et al. The role of initial monitoring of routine biochemical nutritional markers in critically ill children. J Nutr Biochem 2006;17:57-62. [Crossref] [PubMed]
- Protein. In: Pediatric Nutrition Handbook. Edited by Kleinman RE, 6th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009:325-42.
- Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 2009;12:78-85. [Crossref] [PubMed]
- Davis TA, Suryawan A, O'Connor PMJ, et al. Insulin and amino acids independently stimulate translation initiation in neonatal muscle. Faseb Journal 2001;15:A268.
- Davis TA, Suryawan A, Orellana RA, et al. Postnatal ontogeny of skeletal muscle protein synthesis in pigs. J Anim Sci 2008;86:E13-E18. [Crossref] [PubMed]
- El-Kadi SW, Suryawan A, Gazzaneo MC, et al. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab 2012;302:E674-86. [Crossref] [PubMed]
- Hulst JM, van Goudoever JB, Zimmermann LJ, et al. The effect of cumulative energy and protein deficiency on anthropometric parameters in a pediatric ICU population. Clin Nutr 2004;23:1381-9. [Crossref] [PubMed]
- De Bandt JP, Cynober L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. J Nutr 2006;136:308S-313S. [Crossref] [PubMed]
- Hasselgren PO, Fischer JE. Sepsis: stimulation of energy-dependent protein breakdown resulting in protein loss in skeletal muscle. World J Surg 1998;22:203-8. [Crossref] [PubMed]
- Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. Am J Clin Nutr 2012;96:591-600. [Crossref] [PubMed]
- Schreiber G, Howlett G, Nagashima M, et al. The acute phase response of plasma protein synthesis during experimental inflammation. J Biol Chem 1982;257:10271-7. [Crossref] [PubMed]
- Verbruggen SC, Schierbeek H, Coss-Bu J, et al. Albumin synthesis rates in post-surgical infants and septic adolescents; influence of amino acids, energy, and insulin. Clin Nutr 2011;30:469-77. [Crossref] [PubMed]
- Argaman Z, Young VR, Noviski N, et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med 2003;31:591-7. [Crossref] [PubMed]
- de Betue CT, van Waardenburg DA, Deutz NE, et al. Increased protein-energy intake promotes anabolism in critically ill infants with viral bronchiolitis: a double-blind randomised controlled trial. Arch Dis Child 2011;96:817-22. [Crossref] [PubMed]
- Pierro A, Eaton S. Metabolism and nutrition in the surgical neonate. Semin Pediatr Surg 2008;17:276-84. [Crossref] [PubMed]
- Verbruggen SC, Coss-Bu J, Wu M, et al. Current recommended parenteral protein intakes do not support protein synthesis in critically ill septic, insulin-resistant adolescents with tight glucose control. Crit Care Med 2011;39:2518-25. [Crossref] [PubMed]
- Evans WJ, Morley JE, Argiles J, et al. Cachexia: a new definition. Clin Nutr 2008;27:793-9. [Crossref] [PubMed]
- Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care 2005;8:61-5. [Crossref] [PubMed]
- van Waardenburg DA, Deutz NE, Hoos MB, et al. Assessment of whole body protein metabolism in critically ill children: can we use the [15N]glycine single oral dose method? Clin Nutr 2004;23:153-60. [Crossref] [PubMed]
- Jespersen JG, Nedergaard A, Reitelseder S, et al. Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically ill patients. PLoS One 2011;6:e18090. [Crossref] [PubMed]
- Teng Chung T, Hinds CJ. Treatment with GH and IGF-1 in critical illness. Crit Care Clin 2006;22:29-40. vi. [Crossref] [PubMed]
- Dioguardi FS. Wasting and the substrate-to-energy controlled pathway: a role for insulin resistance and amino acids. Am J Cardiol 2004;93:6A-12A. [Crossref] [PubMed]
- Tang JE, Moore DR, Kujbida GW, et al. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107:987-92. [Crossref] [PubMed]
- Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 2007;293:E453-E459. [Crossref] [PubMed]
- Orellana RA, Suryawan A, Kimball SR, et al. Insulin signaling in skeletal muscle and liver of neonatal pigs during endotoxemia. Pediatr Res 2008;64:505-510. [Crossref] [PubMed]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 2005;37:1974-84. [Crossref] [PubMed]
- Suryawan A, Jeyapalan AS, Orellana RA, et al. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 2008;295:E868-75. [Crossref] [PubMed]
- Du J, Wang X, Miereles C, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 2004;113:115-23. [Crossref] [PubMed]
- McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell 2004;119:907-10. [Crossref] [PubMed]
- Orellana RA, Suryawan A, Wilson FA, et al. Development aggravates the severity of skeletal muscle catabolism induced by endotoxemia in neonatal pigs. Am J Physiol Regul Integr Comp Physiol 2012;302:R682-90. [Crossref] [PubMed]
- Suryawan A, Nguyen HV, Bush JA, et al. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 2001;281:E908-E915. [Crossref] [PubMed]
- Hernandez-García A, Manjarin R, Suryawan A, et al. Amino acids, independent of insulin, attenuate skeletal muscle autophagy in neonatal pigs during endotoxemia. Pediatr Res 2016;80:448-51. [Crossref] [PubMed]
- Rudrappa SS, Wilkinson DJ, Greenhaff PL, et al. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review. Front Physiol 2016;7:361. [Crossref] [PubMed]
- Manjarín R, Suryawan A, Koo SJ, et al. Insulin modulates energy and substrate sensing and protein catabolism induced by chronic peritonitis in skeletal muscle of neonatal pigs. Pediatr Res 2016;80:744-52. [Crossref] [PubMed]
- Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 1995;95:811-9. [Crossref] [PubMed]
- Gore DC, Wolf SE, Sanford AP, et al. Extremity hyperinsulinemia stimulates muscle protein synthesis in severely injured patients. Am J Physiol Endocrinol Metab 2004;286:E529-E534. [Crossref] [PubMed]
- Greenhaff PL, Karagounis LG, Peirce N, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 2008;295:E595-E604. [Crossref] [PubMed]
- Whyte MB, Jackson NC, Shojaee-Moradie F, et al. Metabolic effects of intensive insulin therapy in critically ill patients. Am J Physiol Endocrinol Metab 2010;298:E697-705. [Crossref] [PubMed]
- Hotamisligil GS, Peraldi P, Budavari A, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996;271:665-8. [Crossref] [PubMed]
- Bruins MJ, Deutz NE, Soeters PB. Aspects of organ protein, amino acid and glucose metabolism in a porcine model of hypermetabolic sepsis. Clin Sci (Lond) 2003;104:127-41. [Crossref] [PubMed]
- del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol 1999;276:E849-55. [PubMed]
- Orellana RA, Kimball SR, Suryawan A, et al. Insulin stimulates muscle protein synthesis in neonates during endotoxemia despite repression of translation initiation. Am J Physiol Endocrinol Metab 2007;292:E629-E636. [Crossref] [PubMed]
- Perseghin G, Petersen K, Shulman GI. Cellular mechanism of insulin resistance: potential links with inflammation. Int J Obes Relat Metab Disord 2003;27:S6-11. [Crossref] [PubMed]
- Hu Z, Wang H, Lee IH, et al. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest 2009;119:3059-69. [Crossref] [PubMed]
- Navegantes LC, Migliorini RH, do Carmo Kettelhut I. Adrenergic control of protein metabolism in skeletal muscle. Curr Opin Clin Nutr Metab Care 2002;5:281-6. [Crossref] [PubMed]
- Young VR, Bier DM. Amino acid requirements in the adult human: how well do we know them? J Nutr 1987;117:1484-7. [Crossref] [PubMed]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids 2009;37:1-17. [Crossref] [PubMed]
- Demling R. The use of anabolic agents in catabolic states. J Burns Wounds 2007;6:e2. [PubMed]
- Beale RJ, Sherry T, Lei K, et al. Early enteral supplementation with key pharmaconutrients improves Sequential Organ Failure Assessment score in critically ill patients with sepsis: outcome of a randomized, controlled, double-blind trial. Crit Care Med 2008;36:131-44. [Crossref] [PubMed]
- Kovarik M, Muthny T, Sispera L, et al. Effects of β-hydroxy-β-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J Physiol Biochem 2010;66:311-9. [Crossref] [PubMed]
- Wilson FA, Suryawan A, Gazzaneo MC, et al. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 2010;140:264-70. [Crossref] [PubMed]
- Coss-Bu JA, Jefferson LS, Levy ML, et al. Nutrition requirements in patients with toxic epidermal necrolysis. Nutr Clin Pract 1997;12:81-4. [Crossref] [PubMed]
- Kreymann G, DeLegge MH, Luft G, et al. The ratio of energy expenditure to nitrogen loss in diverse patient groups--a systematic review. Clin Nutr 2012;31:168-75. [Crossref] [PubMed]
- Maxvold NJ, Smoyer WE, Custer JR, et al. Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Crit Care Med 2000;28:1161-5. [Crossref] [PubMed]
- Zappitelli M, Goldstein SL, Symons JM, et al. Protein and calorie prescription for children and young adults receiving continuous renal replacement therapy: a report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Crit Care Med 2008;36:3239-45. [Crossref] [PubMed]
- Zappitelli M, Juarez M, Castillo L, et al. Continuous renal replacement therapy amino acid, trace metal and folate clearance in critically ill children. Intensive Care Med 2009;35:698-706. [Crossref] [PubMed]
- Paddon-Jones D, Sheffield-Moore M, Urban RJ, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 2004;89:4351-8. [Crossref] [PubMed]
- Orellana RA, Kyle UG, Coss Bu JA. Nutritional Assessment and Feeding in the ICU. In: Stockwell JA, Preissig CM. editors. Comprehensive Critical Care: Pediatric. Mount Prospect, IL: Society of Critical Care Medicine, 2012:931-48.
- Fujita Y, Okuda T, Rikimaru T, et al. Studies of nitrogen balance in male highlanders in Papua New Guinea. J Nutr 1986;116:536-44. [Crossref] [PubMed]
- Liu B, Qian SB. Translational regulation in nutrigenomics. Adv Nutr 2011;2:511-9. [Crossref] [PubMed]
Cite this article as: Orellana RA, Coss-Bu JA. Metabolic alterations in the critically ill child: a narrative review. Pediatr Med 2021;4:8.