Refeeding syndrome and other related issues in the paediatric intensive care unit
Introduction
Refeeding syndrome describes a potentially fatal physiological response to the increase in energy (carbohydrate) from any source including: intravenous (IV) dextrose (e.g., 5% dextrose solution), oral food, enteral nutrition (EN) or parenteral nutrition (PN) following a period of starvation or severely reduced energy intake (1), reflecting a change from catabolism to anabolism following the release of insulin (2). The most common symptoms attributed to refeeding syndrome include electrolyte abnormalities (e.g., hypokalemia, hypomagnesemia, hypophosphatemia), cardiac and respiratory failure. In hospitalised children, refeeding syndrome is commonly reported to occur following a period of inadequate nutrition of 7–10 days duration (3).
Definition of refeeding syndrome
Until recently, there has been no consensus based definitions for refeeding syndrome. The American Society for Parenteral and Enteral Nutrition (ASPEN) committee and clinical practice task force comprising of dietitians, pharmacists, clinicians and nurses developed consensus based recommendations for screening and managing patients at risk of, or who have developed, refeeding syndrome (4). ASPEN’s definition of refeeding syndrome is “a measurable reduction in levels of one or any combination of phosphorus, potassium, and/or magnesium, or the manifestation of thiamine deficiency, developing shortly (hours or days) after the initiation of calorie provision to an individual who has been exposed to a substantial period of undernourishment”. ASPEN proposed the following diagnostic criteria for refeeding syndrome as being (4):
- A reduction in serum levels in one or any of the electrolytes, phosphorus, potassium or magnesium by 10–20% (mild refeeding syndrome), 20–30% (moderate refeeding syndrome), or >30% (severe refeeding syndrome), or organ dysfunction results from a decrease in any of these and/or as a result of thiamine deficiency (severe refeeding syndrome).
- Combined with this occurrence within 5 days of recommencing or significantly increasing energy provision.
Pathophysiology
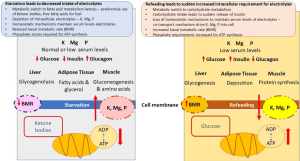
Refeeding syndrome is a response to rapidly changing hormonal pattern and downstream effects on metabolic pathways. During starvation, the basal metabolic rate may be reduced by 20–25%, in addition to metabolic conservation strategies involving switching from using glucose as the primary fuel to that of ketone bodies and the downregulation of pathways including gluconeogenesis (5). During periods of starvation, homeostatic mechanisms ensure serum concentrations of electrolytes such as magnesium, potassium and phosphorous are maintained by depleting intracellular ion stores, as well as mechanisms to reduce renal excretion of electrolytes (2,5).
On refeeding, with increased amount of carbohydrate, hormone levels of glucagon rapidly decrease with glycaemia and subsequent release of the anabolic hormone insulin. Increasing insulin levels drive phosphorus and potassium intracellularly to meet increased demand (e.g., phosphorylation of glucose as a result of glycolysis) and also as a result of stimulation of the sodium-potassium adenosine triphosphate (ATP)ase transporter promoting active transport of glucose and potassium and phosphorus into the cell, where phosphorus is also required for phosphorylation of glucose as glycolysis is stimulated. Phosphate is an essential component of ATP, malnutrition may lead to phosphate depletion leading to increased risk of respiratory failure (4). Thiamine deficiency may also occur as a result of malnutrition. Thiamine requirements are significantly increased during the transition from starvation to feeding, as it is involved (co-factor) for glucose dependent metabolic pathways (6). These concomitant metabolic processes result in a precipitous drop in serum levels of these electrolytes (4). Refeeding syndrome occurs as a result of functional serum deficits of these minerals in addition to a rapid change in basal metabolic rate (2,5). Fluid overload arising from hyperinsulinemic decrease in renal excretion of sodium and water leading to pulmonary oedema and congestive cardiac failure (Figure 1) (7).
Signs and symptoms of refeeding syndrome
Although the clinical signs of varied hallmark characteristics are (I) electrolyte abnormalities (hypokalaemia, hypomagnesaemia, hypophosphatemia and sodium retention) and thiamine deficiency (II) hyperglycaemia, (III) cardiac—arrhythmias, heart failure, (IV) respiratory—respiratory failure, intercostal and diaphragm muscle failure, failure to wean from the ventilatory, (V) haematology—anaemia, (VI) immunological—immune dysfunction, (VII) neurological—Wernicke’s encephalopathy, and (VIII) musculoskeletal—weakness and rhabdomyolysis (4,7).
Consensus criteria for identifying paediatric patients at risk of refeeding syndrome
Those most at risk for refeeding syndrome are severely malnourished children with starvation physiology (8), as well as children with inflammatory bowel disease (9,10), anorexia nervosa (11), preterm infants (12), and haematological cancers (13). Yet despite refeeding syndrome being considered an issue for critically ill children, there is an absence of evidence to guide medical management. ASPEN has proposed characteristics and associated diagnosis for children at risk of refeeding syndrome (4) (Tables 1,2).
Table 1
| Section 1: paediatric diagnosis requiring PICU admission associated with an increased risk of refeeding syndrome |
| 1. Major stressors or surgery without nutrition for prolonged periods of time |
| 2. Oncology—solid tumours/haematological cancers |
| 3. Post-operative patients with complications |
| 4. Malabsorption e.g., short bowel syndrome, pancreatitis, pyloric stenosis |
| 5. Refugees or patients from disadvantaged countries—including those with late diagnosis of chronic medical conditions e.g., congenital heart disease |
| 6. Premature infant or small for gestational age/intrauterine growth retardation |
| 7. Anorexia nervosa |
ASPEN, American Society for Parenteral and Enteral Nutrition; PICU, paediatric intensive care unit.
Table 2
| Section 2 | Mild risk—3 risk criteria required | Moderate risk—2 risk criteria required | Severe risk—1 risk criteria required |
|---|---|---|---|
| Weight for length z-scores (<24 months) | –1 to –1.9 z-scores—change from baseline | –2 to –2.9 z-scores—change from baseline | >–3 z-scores—change from baseline |
| Body mass index for age z-scores >2 years | <75% of normal for expected weight gain | <50% of normal for expected weight gain | <25% of normal for expected weight gain |
| Weight loss | 3–5 consecutive days of protein or energy intake <75% of estimated requirements | 5–7 consecutive days of protein or energy intake <75% of estimated requirements | >7 consecutive days of protein or energy intake <75% of estimated requirements |
| Energy intake | Mildly abnormal or decreased to 25% below lower limit of normal | Moderate/significantly abnormal or decreased to 25–50% below lower limit of normal | – |
| Abnormal prefeeding serum potassium, phosphorus, magnesium | Mild disease | Moderate disease | Severe disease |
| Diagnosis—section 1 | Evidence of mild loss | Evidence of moderate loss | Evidence of severe loss |
| Loss of subcutaneous fat | –1 to –1.9 z-scores | –2 to –2.9 z-scores | >–3 z-scores |
ASPEN, American Society for Parenteral and Enteral Nutrition.
Incidence of refeeding syndrome in critical illness
Paediatric critical illness
There are no studies considering the management of refeeding syndrome in children during critical illness. Only one study showed that the incidence of refeeding syndrome in critically ill children on PN was of about 9%, according to the criteria used in the institution (14). ASPEN provides management and treatment strategies for paediatric patients, however, they are not age specific and arguably may result in significant overfeeding of older children e.g., starting at a glucose infusion rate of 4–6 mg/kg/min (5.8–8.6 g/kg/day) and advancing by 1–2 mg/kg/min/per day aiming for a maximum glucose rate of 14–18 mg/kg/min (20.1–25.9 g/kg/day) (4). These starting and goal rates are significantly higher than those recommenced for critically ill children by the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) (15) and the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) (16). For critically ill children, recent guidelines recommend energy intake should not exceed resting energy expenditure (REE) during the acute phase of critical illness (15-17). As this recommended amount is significantly lower than those for children in a normal ward setting, the incidence of refeeding syndrome in critically ill children may also be reduced. Prospective studies are needed to confirm this.
Neonatal critical illness
Neonates were excluded from the ASPEN consensus based recommendations for the avoidance and treatment of refeeding syndrome (4). However, the committee did recognise that neonates at risk of refeeding syndrome include those who are small for gestational age; including those inter uterine growth restriction arising from maternal complications, elevated uterine artery resistance index, extreme prematurity e.g., <28 weeks gestational age, very low birth weight and weight for age or length for age of <–2 z-scores (4). As infants arguably comprise of up to 50% of the paediatric intensive care unit (PICU) population (18,19), recognising those at risk and implementing appropriate management strategies is important and may differ from those of older children. In 2014, approximately 15 million preterm babies were born, equating to 11% of all live births worldwide, with a range of 9% in European and more than 80% in Asia and sub-Saharan Africa, where 45% of all global live births occur (20). More recently, refeeding syndrome has been described in inter uterine growth retarded term infants and preterm infants (21-28), with a number of hypothesis including a mechanism termed, placental interrupted feeding syndrome of the preterm infant (27). Medical advances, including the use of early aggressive PN with up to 3.5 g/kg per day of amino acids in infants <32 weeks gestational age (29), have significantly improved nutritional outcomes conserving intrauterine growth patterns (30). However, the unintended consequences associated with high early amino acid and glucose use (following sudden abrupt cessation of maternal nutrient supply) are hypophosphatemia, hyperkalaemia (27,31), zinc deficiency (32), and dysregulated acid-base homeostasis during the first week of life (27,31). This has been likened to refeeding syndrome with similar physiological mechanisms as those occurring in other conditions where there is nutritional deprivation e.g., severe oedematous malnutrition (21-28). Management strategies to ameliorate this have included an increased amount of phosphorus (27) and zinc (32) when providing early aggressive nutrition support.
Studies describing electrolyte imbalance in critically ill children
Electrolyte imbalances in critically ill children are common and the presence of hypophosphatemia, hypomagnesaemia and hypokalaemia, alone does not necessarily mean refeeding syndrome is present. There are many other causes, which is of particular relevance to critically ill children, as in multiple pharmacopeia that are used to provide organ support such as e.g., inotropes, diuretics, insulin (1,2) (Table 3). Potassium plays an essential role in the excitability of skeletal, cardiac and smooth muscle (33). Hypokalaemia affects 40% of critically ill children with 16% experiencing moderate to severe hypokalaemia (<3.0 mmol/L), with severity associated with increased risk of mortality (34). Magnesium is an essential co-factor for over 300 enzymes, including ones associated ATP activity (35). In children, hypomagnesemia was associated with higher mortality rate and duration of PICU length of stay compared to those with normal serum levels (36), although other studies have not found a similar association with serum levels influenced by diuretic use (37). There are a number of studies considering the incidence of hypophosphatemia in critically ill children (38-42), affecting up to 42% of children on PICU admission and was associated with an increased risk of length of stay. Risk factors associated with hypophosphatemia were malnutrition and the use of furosemide, dopamine, steroids and β2 agonist (43).
Table 3
| Hypophosphatemia | Hypomagnesaemia | Hypokalaemia |
|---|---|---|
| Cellular phosphate redistribution | Cellular magnesium redistribution | Cellular potassium redistribution |
| Insulin administration | Inotropes | Inotropes |
| Metabolic or respiratory alkalosis | “Hungry” bone syndrome | Glucose and insulin |
| Intravenous (IV) glucose | Drugs | Vitamin B12 therapy |
| Increased losses or poor intake | Aminoglycosides | Rapidly growing tumours |
| Inappropriate feed or inadequate intake | b2-adrenergic agonists | Gastrointestinal loss of potassium |
| High output stoma | Cyclosporin and tacrolimus | Inappropriate feed or inadequate intake |
| Diarrhoea | Cytotoxic | High output stoma |
| Phosphate binding medications e.g., proton pump inhibitors | Diuretics | Diarrhoea |
| Prematurity | Pamidronate, pentamidine, amphotericin B, foscarnet | Pyloric stenosis |
| Renal tubular phosphate loss | Proton pump inhibitors | Vomiting |
| Post-trauma | Increased renal loss of magnesium | Renal potassium loss |
| Fanconi syndrome | Postrenal transplantation | Mineralocorticoid excess conditions |
| Hypophosphatemic osteopenia of prematurity | Cardiac surgery | Cushing’s syndrome |
| Chronic diuretic use | Continuous renal replacement therapy | Renal tubule mechanisms |
| Oncogenic hypophosphatemia | Prematurity | Alkalemia |
| Others | Poor magnesium intake | Carbonic dehydratase inhibitors |
| Hyperparathyroidism or parathyroid hormone-related peptide release | Inappropriate feed or inadequate intake | Fanconi syndrome |
| Liver disease | High output stoma | Severe hypomagnesemia |
| Septicaemia | Diarrhoea | Diuretics |
| Miscellaneous | Renal tubular acidosis | |
| Diabetes mellitus | ||
| Hyperaldosteronism | ||
| Hypercalcemia | ||
| Hyperthyroidism |
The prevalence of thiamine deficiency in critically ill children
Thiamine is involved in a number of intermediate metabolic pathways associated with energy production including converting pyruvate from glucose into acetyl co-enzyme A for entry into the Krebs cycle, during thiamine deficiency alters intermediate metabolism resulting in lactic acidosis (44). Serum studies of thiamine in critical illness report 12.5–32% of patients have low thiamine levels, associated factors is the magnitude of acute phase inflammatory response (44), malnutrition (45), but not diuretic use (46) and was associated with reduced mortality risk in malnourished patients (47) but no effect was demonstrated in well-nourished patients (45,48). Four of the case studies focused on thiamine deficiency and lactic acidosis, reporting cases of symptom resolution following the supplementation of thiamine, limiting lactate production by limiting pyruvate dehydrogenase activity in septic shock (48), extracorporeal membrane oxygenation (49), haematological malignancies (50), and following cardiac surgery (51).
Screening, assessment and management of critically ill children at risk of refeeding syndrome
Assessing risk
Despite the lack of scientific data, the patients most at risk for refeeding syndrome seem to be malnourished children and those with reduced or restricted intake for more than 7–10 days (7). Prior to commencing nutrition support it may be useful for clinicians to consider the following screening questions:
- Is the children at risk of refeeding syndrome e.g., malnutrition <–2 weight-of-length z-scores or history of poor intake for 7–10 days?
- Are serum electrolytes low or on the lower end of the normal range before nutrition support has been commenced?
- Is the child on multiple pharmacopeia including diuretics and inotropic support?
Nutrition assessment
Careful screening of these patients through routine nutritional assessment on admission to PICU is essential (15), using weight-for-length z-score, body mass index-for-age, mid upper arm circumference for age using established malnutrition cut-off criteria e.g., <–2 weight-for-length z-score as those with moderate malnutrition. Recent weight loss should be documented, (if this is not possible asking caregivers for a visual assessment) along with a recent diet history are all essential to assess refeeding syndrome risk before commencing any nutrition support.
Nutrition considerations for critically ill children at risk of refeeding syndrome
During the last decades, there has been increasing recognition that during the early acute phase of critical illness cautious nutrition support should be provided, with energy intake not exceeding REE, as measured by indirect calorimetry or estimated using Schofield equation (15,52), which is then increased accordingly during the stable and rehabilitative phases of critical illness (53,54). The World Health Organization (WHO) management of feeding of severe acute malnutrition follows very similar principles (8) to those recommended by Critical Care Societies (15,17,55,56) with cautious feeding with slow graded increase over a number of days (15,17,55,56) (Figure 2) (8). Particularly as mortality and morbidity are reportedly reduced with lower energy and protein intake during the acute phase (57,58). For energy, the intake should not exceed the REE and for protein an intake of 1.5 g/kg/day is recommended during the acute phase (15).
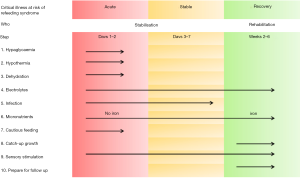
In the acute phase of disease, excessive use of glucose should be avoided [maximum 5 mg/kg/min (7.2 g/kg/day)] because of the risk of hyperglycaemia due to the presence of insulin resistance in critically ill children (59), in addition to recommendations for energy (i.e., <REE) (16), amino acid (60) and protein (i.e., 1.5 g/kg/day) (58) requirements should also not be exceeded. If PN is required, it should be done so in line with ESPGHAN recommendations for energy, glucose and lipids to be administered parenterally according to age, weight and stage of disease in critically ill children (16,59,61). Micronutrient requirements during critical illness are poorly described with current recommendations suggesting they should be provided in sufficient amounts to meet reference nutrient intakes for age (62,63). However, in the context of refeeding syndrome, in those at-risk children, additional thiamine may be required with a recommended dose of 2 mg/kg (up to a maximum of 100–200 mg/day), for up to 5–7 days in addition to a multivitamin (4).
Management of children at risk of refeeding syndrome
The ESPNIC Metabolism, Endocrinology and Nutrition section provides recommendations with regards to assessment, use of nutritional algorithms, macronutrient requirements and feeding mode and type during guidance on nutritional support during critical illness, which can be adapted for children at risk of refeeding syndrome (Table 4) (15). To guide the timing and management of nutritional support in the PICU, the presence of a nutrition team including a dedicated dietitian is recommended. Before starting nutritional support, nutrition assessment should be completed, in addition to the monitoring and correction of any electrolytes, particularly potassium, phosphorus and magnesium is essential, along with multivitamin and thiamine supplementation. For children where serum electrolyte levels are difficult to correct or drop precipitously, where no other cause can be attributed, a reduction in energy (including dextrose containing fluid) and protein intake of up to 50% may be required for 24–48 hours. In children where serum electrolyte levels are severely or life threateningly low, nutrition support may need to be withheld for 24–48 hours in order to replete these serum levels and restore homoeostasis (4). Once nutrition support is able to be established, increasing nutrition support towards nutrition goals should be possible and increased in a stepwise manner, according to the phase of critical illness (15,52,54). Depending on nutritional status prior to admission in PICU, nutrition support may need to be continued for longer into the recovery phase until sufficient oral intake is consistently achieved to support physical and nutritional rehabilitation (54).
Table 4
| Aspect of care | Acute phase | Stable phase | Recovery phase |
|---|---|---|---|
| Energy—using Schofield equation for weight | <REE | 1.3–1.5× REE | 2× REE |
| Protein (g/kg/day) | 1–2 | 2–3 | 3–4 |
| Carbohydrates mg/kg/min (g/kg/day) | |||
| Newborn | 2.5–5 (3.6–7.2) | 5–10 (7.2–14) | 5–10 (7.2–14) |
| 28 d–10 kg | 2–4 (2.9 –5.8) | 4–6 (5.8–8.6) | 6–10 (8.6–14) |
| 11–30 kg | 1.5–2.5 (1.4–2.2) | 2–4 (2.8–5.8) | 3–6 (4.3–8.6) |
| 31–45 kg | 1–1.5 (1.4–2.2) | 1.5–3 (2.2–4.3) | 3–4 (4.3–5.8) |
| >45 kg | 0.5–1 (0.7–1.4) | 1–2 (1.4–2.9) | 2–3 (2.9–4.3) |
| Electrolytes | |||
| Monitor—serum magnesium, phosphate, potassium | 12 hourly | Daily | As required |
| Replace—electrolytes | Replace and replete low levels of electrolytes based on standards of care | Replace and replete low levels of electrolytes based on standards of care | |
| If electrolytes are difficult to correct or precipitously drop after nutrition support is commenced, reduce calorie/protein intake by 50% for 24 hours and then advance dextrose/calories by approximately 33% of goal every 24–48 hours | Aim to increase nutrition support to meet requirements including sufficient calories and protein to support recovery and rehabilitation | ||
| Cessation of nutrition support may be considered for 24–48 hours if electrolyte levels are severely/life threateningly low or continuing to drop precipitously low despite replacement | |||
| Thiamine and micronutrients | In at risk children, before feeding or IV fluids containing dextrose, is commenced provide 2 mg/kg to a maximum of 100–200 mg/day | For children receiving oral nutrition provide age appropriate oral/enteral multivitamin supplements once daily for 10 days or longer based on nutritional deficit | |
| Continue thiamine supplementation for 5–7 days or longer in children who are severely malnourished | |||
| Routine serum thiamine levels are unlikely to be useful | |||
| For children receiving oral nutrition provide age appropriate oral/enteral multivitamin supplements once daily for 10 days or longer based on nutritional deficit | |||
| Withholding PN should be considered for up to 1 week in critically ill children. However micronutrient injectables including thiamine, may be given intravenously during this time if oral enteral nutritional supplementation is not possible | |||
| Monitoring and long term care | Establish nutrition goals for long term nutrition recovery and rehabilitation considering short terms energy and protein goals | Establish nutrition goals for long term nutrition recovery and rehabilitation considering energy and protein goals | |
| Monitor vital signs are per unit standard including cardiorespiratory monitoring for children who are unstable or those with severe electrolyte disturbances | Energy from protein should be given at 10–15% to support lean body mass acquisition | ||
| Daily weights or monitoring of fluid balance should be completed | Micronutrient supplementation particularly zinc may be required to support catch up weight gain and growth | ||
REE, resting energy expenditure; PN, parenteral nutrition.
Conclusions
There is a paucity of evidence on the management of refeeding syndrome in critically ill children. On admission in PICU, nutrition status should be assessed, including classifying anthropometrical status and taking a diet history. For children with low serum levels of electrolytes, imbalances should be corrected to near normal levels before nutrition support is commenced, along with micronutrient supplementation of multivitamins and thiamine. However, as recent guidelines recommend energy intake should not exceed REE during the acute phase of critical illness, it is likely there is a significantly lower incidence of refeeding syndrome amongst critically ill children hospitalised in the PICU than children in a normal ward setting, although prospective studies would be needed to confirm this.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyvonne Tume, Frederic Valla and Sascha Verbruggen) for the series “Nutrition in the Critically Ill Child” published in Pediatric Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-59). The series “Nutrition in the Critically Ill Child” was commissioned by the editorial office without any funding or sponsorship. CJC reports non-financial support from Baxter, non-financial support from Nutricia, outside the submitted work. CM reports non-financial support from Baxter, non-financial support from Nutricia, outside the submitted work. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koekkoek WAC, Van Zanten ARH. Is refeeding syndrome relevant for critically ill patients? Curr Opin Clin Nutr Metab Care 2018;21:130-7. [Crossref] [PubMed]
- Crook MA. Refeeding syndrome: problems with definition and management. Nutrition 2014;30:1448-55. [Crossref] [PubMed]
- Fuentebella J, Kerner JA. Refeeding syndrome. Pediatr Clin North Am 2009;56:1201-10. [Crossref] [PubMed]
- da Silva JSV, Seres DS, Sabino K, et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr Clin Pract 2020;35:178-95. [Crossref] [PubMed]
- Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ 2008;336:1495-8. [Crossref] [PubMed]
- Schenk G, Duggleby RG, Nixon PF. Properties and functions of the thiamin diphosphate dependent enzyme transketolase. Int J Biochem Cell Biol 1998;30:1297-318. [Crossref] [PubMed]
- Byrnes MC, Stangenes J. Refeeding in the ICU: an adult and pediatric problem. Curr Opin Clin Nutr Metab Care 2011;14:186-92. [Crossref] [PubMed]
- World Health Organization. Management of severe malnutrition: a manual for physicians and other senior health workers. Geneva: WHO, 1999.
- Afzal NA, Addai S, Fagbemi A, et al. Refeeding syndrome with enteral nutrition in children: a case report, literature review and clinical guidelines. Clin Nutr 2002;21:515-20. [Crossref] [PubMed]
- Akobeng AK, Thomas AG. Refeeding syndrome following exclusive enteral nutritional treatment in Crohn disease. J Pediatr Gastroenterol Nutr 2010;51:364-6. [PubMed]
- Bargiacchi A, Clarke J, Paulsen A, et al. Refeeding in anorexia nervosa. Eur J Pediatr 2019;178:413-22. [Crossref] [PubMed]
- Pająk A, Królak-Olejnik B, Szafrańska A. Early hypophosphatemia in very low birth weight preterm infants. Adv Clin Exp Med 2018;27:841-7. [Crossref] [PubMed]
- Jahn HK, Barraclough S, Currell S, et al. Febrile neutropenia and refeeding syndrome. Arch Dis Child Educ Pract Ed 2016;101:296-303. [Crossref] [PubMed]
- Dunn RL, Stettler N, Mascarenhas MR. Refeeding syndrome in hospitalized pediatric patients. Nutr Clin Pract 2003;18:327-32. [Crossref] [PubMed]
- Tume LN, Valla FV, Joosten K, et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med 2020;46:411-25. [Crossref] [PubMed]
- Joosten K, Embleton N, Yan W, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: energy. Clin Nutr 2018;37:2309-14. [Crossref] [PubMed]
- Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med 2020;46:10-67. [Crossref] [PubMed]
- Kanthimathinathan HK, Plunkett A, Scholefield BR, et al. Trends in long-stay admissions to a UK paediatric intensive care unit. Arch Dis Child 2020;105:558-62. [Crossref] [PubMed]
- Davis P, Stutchfield C, Evans TA, et al. Increasing admissions to paediatric intensive care units in England and Wales: more than just rising a birth rate. Arch Dis Child 2018;103:341-5. [Crossref] [PubMed]
- Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37-46. [Crossref] [PubMed]
- Igarashi A, Okuno T, Ohta G, et al. Risk factors for the development of refeeding syndrome-like hypophosphatemia in very low birth weight infants. Dis Markers 2017;2017:9748031 [Crossref] [PubMed]
- Boubred F, Herlenius E, Bartocci M, et al. Extremely preterm infants who are small for gestational age have a high risk of early hypophosphatemia and hypokalemia. Acta Paediatr 2015;104:1077-83. [Crossref] [PubMed]
- Ross JR, Taylor SN. Hyperinsulinemia has prominent role in refeeding syndrome pathophysiology. J Perinatol 2014;34:247-8. [Crossref] [PubMed]
- Hakan N, Aydin M, Dilli D, et al. Transient hyperinsulinemia may be responsible from electrolyte abnormalities of refeeding syndrome seen in very low birth weight infants with intrauterine growth-restriction. J Perinatol 2014;34:247. [Crossref] [PubMed]
- Ross JR, Finch C, Ebeling M, et al. Refeeding syndrome in very-low-birth-weight intrauterine growth-restricted neonates. J Perinatol 2013;33:717-20. [Crossref] [PubMed]
- Moltu SJ, Strommen K, Blakstad EW, et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia--a randomized, controlled trial. Clin Nutr 2013;32:207-12. [Crossref] [PubMed]
- Bonsante F, Iacobelli S, Latorre G, et al. Initial amino acid intake influences phosphorus and calcium homeostasis in preterm infants--it is time to change the composition of the early parenteral nutrition. PLoS One 2013;8:e72880 [Crossref] [PubMed]
- Mizumoto H, Mikami M, Oda H, et al. Refeeding syndrome in a small-for-dates micro-preemie receiving early parenteral nutrition. Pediatr Int 2012;54:715-7. [Crossref] [PubMed]
- Embleton ND, van den Akker CHP. Protein intakes to optimize outcomes for preterm infants. Semin Perinatol 2019;43:151154 [Crossref] [PubMed]
- Andrews ET, Ashton JJ, Pearson F, et al. Early postnatal growth failure in preterm infants is not inevitable. Arch Dis Child Fetal Neonatal Ed 2019;104:F235-41. [Crossref] [PubMed]
- Bonsante F, Gouyon JB, Robillard PY, et al. Early optimal parenteral nutrition and metabolic acidosis in very preterm infants. PLoS One 2017;12:e0186936 [Crossref] [PubMed]
- Terrin G, Boscarino G, Di Chiara M, et al. Nutritional intake influences zinc levels in preterm newborns: an observational study. Nutrients 2020;12:529. [Crossref] [PubMed]
- Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press 2010;8:72-81. [Crossref] [PubMed]
- Cummings BM, Macklin EA, Yager PH, et al. Potassium abnormalities in a pediatric intensive care unit: frequency and severity. J Intensive Care Med 2014;29:269-74. [Crossref] [PubMed]
- Jiang P, Lv Q, Lai T, et al. Does Hypomagnesemia impact on the outcome of patients admitted to the intensive care unit? A systematic review and meta-analysis. Shock 2017;47:288-95. [Crossref] [PubMed]
- Ong C, Han WM, Wong JJ, et al. Nutrition biomarkers and clinical outcomes in critically ill children: a critical appraisal of the literature. Clin Nutr 2014;33:191-7. [Crossref] [PubMed]
- Haque A, Saleem AF. On admission hypomagnesemia in critically ill children: Risk factors and outcome. Indian J Pediatr 2009;76:1227-30. [Crossref] [PubMed]
- Santana e Meneses JF, Leite HP, de Carvalho WB, et al. Hypophosphatemia in critically ill children: prevalence and associated risk factors. Pediatr Crit Care Med 2009;10:234-8. [Crossref] [PubMed]
- de Menezes FS, Leite HP, Fernandez J, et al. Hypophosphatemia in children hospitalized within an intensive care unit. J Intensive Care Med 2006;21:235-9. [Crossref] [PubMed]
- Shah SK, Irshad M, Gupta N, et al. Hypophosphatemia in critically ill children: risk factors, outcome and mechanism. Indian J Pediatr 2016;83:1379-85. [Crossref] [PubMed]
- Kilic O, Demirkol D, Ucsel R, et al. Hypophosphatemia and its clinical implications in critically ill children: a retrospective study. J Crit Care 2012;27:474-9. [Crossref] [PubMed]
- de Menezes FS, Leite HP, Fernandez J, et al. Hypophosphatemia in critically ill children. Rev Hosp Clin Fac Med Sao Paulo 2004;59:306-11. [Crossref] [PubMed]
- El Shazly AN, Soliman DR, Assar EH, et al. Phosphate disturbance in critically ill children: Incidence, associated risk factors and clinical outcomes. Ann Med Surg (Lond) 2017;21:118-23. [Crossref] [PubMed]
- Lima LF, Leite HP, Taddei JA. Low blood thiamine concentrations in children upon admission to the intensive care unit: risk factors and prognostic significance. Am J Clin Nutr 2011;93:57-61. [Crossref] [PubMed]
- Seear M, Lockitch G, Jacobson B, et al. Thiamine, riboflavin, and pyridoxine deficiencies in a population of critically ill children. J Pediatr 1992;121:533-8. [Crossref] [PubMed]
- Shamir R, Dagan O, Abramovitch D, et al. Thiamine deficiency in children with congenital heart disease before and after corrective surgery. JPEN J Parenter Enteral Nutr 2000;24:154-8. [Crossref] [PubMed]
- Leite HP, de Lima LFP, Taddei J, et al. Effect of blood thiamine concentrations on mortality: Influence of nutritional status. Nutrition 2018;48:105-10. [Crossref] [PubMed]
- Weiss SL, Blowey B, Keele L, et al. Matched retrospective cohort study of thiamine to treat persistent hyperlactatemia in pediatric septic shock. Pediatr Crit Care Med 2019;20:e452-6. [Crossref] [PubMed]
- Teagarden AM, Leland BD, Rowan CM, et al. Thiamine deficiency leading to refractory lactic acidosis in a pediatric patient. Case Rep Crit Care 2017;2017:5121032 [Crossref] [PubMed]
- Lerner RK, Pessach I, Rubinstein M, et al. Lactic acidosis as presenting symptom of thiamine deficiency in children with hematologic malignancy. J Pediatr Intensive Care 2017;6:132-5. [PubMed]
- Simalti AK, Joshi R, Aggarwal N, et al. An unusual cause of persisting hyperlactatemia in a neonate undergoing open heart surgery. World J Pediatr Congenit Heart Surg 2015;6:130-4. [Crossref] [PubMed]
- Joosten KF, Kerklaan D, Verbruggen SC. Nutritional support and the role of the stress response in critically ill children. Curr Opin Clin Nutr Metab Care 2016;19:226-33. [PubMed]
- Joosten K, van Puffelen E, Verbruggen S. Optimal nutrition in the paediatric ICU. Curr Opin Clin Nutr Metab Care 2016;19:131-7. [Crossref] [PubMed]
- Joosten KFM, Eveleens RD, Verbruggen S. Nutritional support in the recovery phase of critically ill children. Curr Opin Clin Nutr Metab Care 2019;22:152-8. [Crossref] [PubMed]
- Mehta NM, Compher C. A.S.P.E.N. Board of Directors. A.S.P.E.N. Clinical Guidelines: nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr 2009;33:260-76. [Crossref] [PubMed]
- Mihatsch WA, Braegger C, Bronsky J, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition. Clin Nutr 2018;37:2303-5. [Crossref] [PubMed]
- Vanhorebeek I, Verbruggen S, Casaer MP, et al. Effect of early supplemental parenteral nutrition in the paediatric ICU: a preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir Med 2017;5:475-83. [Crossref] [PubMed]
- Mehta NM, Bechard LJ, Cahill N, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children--an international multicenter cohort study*. Crit Care Med 2012;40:2204-11. [Crossref] [PubMed]
- Mesotten D, Joosten K, van Kempen A, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: carbohydrates. Clin Nutr 2018;37:2337-43. [Crossref] [PubMed]
- van Goudoever JB, Carnielli V, Darmaun D, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Amino acids. Clin Nutr 2018;37:2315-23. [Crossref] [PubMed]
- Lapillonne A, Fidler Mis N, Goulet O, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin Nutr 2018;37:2324-36. [Crossref] [PubMed]
- Dao DT, Anez-Bustillos L, Cho BS, et al. Assessment of micronutrient status in critically ill children: challenges and opportunities. Nutrients 2017;9:1185. [Crossref] [PubMed]
- Marino LV, Valla FV, Beattie RM, et al. Micronutrient status during paediatric critical illness: a scoping review. Clin Nutr 2020; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Marino LV, Jotterand Chaparro C, Moullet C. Refeeding syndrome and other related issues in the paediatric intensive care unit. Pediatr Med 2020;3:15.