
Birth asphyxia-induced brain damage: the long road to optimal reduction and prevention!
Introduction
Neonatal encephalopathy due to perinatal hypoxia-ischemia (HIE) is to date one of the most important causes of an adverse neurodevelopmental outcome and even death: 2 up to 26 neonates per 1,000 live births have an adverse long-term outcome such as cerebral palsy, epilepsy, cognitive impairment and learning difficulties (1-3). Moreover, birth asphyxia is responsible for 25% of the neonatal mortality rate in the world (4).
Etiologically a curtailed perfusion and, consequently, insufficient oxygenation is the most important cause for the injury to the (fetal) brain, the more the neonatal brain has high metabolic demands (5) and high concentrations of fatty acids and free ions leading to excessive free radical formation and other toxic compounds such as peroxynitrite (6,7).
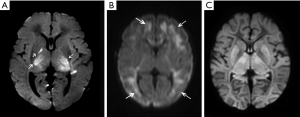
Hypoxia-ischemia around birth starts for 20% of the birth asphyxia cases before birth, for 30% during the delivery and for 35% before and during the birth process. Only 10% of Hypoxia-ischemia develops after birth (8). In the majority of perinatal asphyxia there is an acute hypoxic-ischemic insult and in such situations the deeper brain structures such as brain stem, thalamus and basal ganglia are in particular affected, whereas subacute and chronic hypoxia-ischemia, which happens in about 10% to15% during perinatal asphyxia, induce damage at the borders of the vascular beds of the anterior, middle and posterior cerebral arteries, the so-called watershed damage (9,10). Hypoxic-ischemic insults that lead to a global pattern of injury are relatively rare, probably because in most cases this will result in fetal or intrapartum death, and infants will not undergo MRI examinations (9). See also Figure 1.
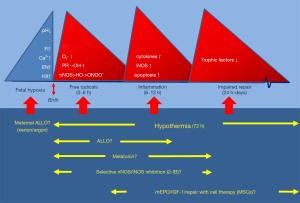
The process of brain damage due to perinatal hypoxia-ischemia is two-fold. During the actual insult, almost always starting before birth, (fetal) hypoxia-ischemia induces an abnormal production of excitatory neurotransmitters and lowering of intra- and extra-cellular pH already leading to neural cell death and accumulation of potentially damaging compounds such as free (pro-) radicals. Metabolization of these compounds after recovery of perfusion and oxygenation after birth can set in motion destructive molecular pathways (see also below) leading to secondary energy failure after an initially recovered oxidative metabolism in the first hours of life (11). This delayed impairment of cerebral oxidative energy metabolism, mostly apparent after 6 to 8 hours after birth, can last up to 72 hours after birth and adds substantial brain damage due to necrosis and apoptosis neuronal cells (12). This postnatal process of cell death can even last for weeks (13).
Moderate hypothermia (HT) is the only neuroprotective therapy which has a proven positive impact on perinatal asphyxia-driven brain damage and will be briefly discussed. At present a large number of pharmaceuticals are under investigation as add-on therapy for HT. This paper summarizes the most promising of these drugs, expected to interrupt the devastating effects of destructive molecular pathways set in motion by perinatal hypoxia-ischemia. The most effective treatment of birth asphyxia is expected to be a combination of HT with consecutive (fetal) pharmacological therapies intervening in one (or more) of the destructive pathways (as described below). Finally it has to be expected that repair of the developing brain will play an increasingly important role in the coming years.
Neuroprotection and repair after perinatal hypoxia-ischemia
Moderate hypothermia
The only proven therapy is moderate hypothermia or HT down to a core temperature of 33.5 °C for 72 hours (14,15). Body temperature should be monitored rectally, transesophageally or nasopharyngeally (16,17). Clinical randomized studies showed that HT lowered adverse outcome at 18–24 month or mortality from about 55% to 60% back to 42% (17,18). Also long-term outcome up to 7 years of age showed that HT decreased cerebral palsy substantially as compared to non-cooled counterparts (19,20). HT should be installed in the first 6 hours after birth, in the so-called therapeutic window, when the energy metabolism is still apparently normal (11). Although the protective mechanism of HT is not yet fully explained and is linked to lowering of free radical formation and to anti-inflammatory and anti-apoptotic properties (21,22), its reducing effect on the hypoxic-ischemic-induced production of excitatory neurotransmitters seem to be important (23). Since neurotransmitter and free radical formation occurs during (fetal) hypoxia and intensifies upon reperfusion and reoxygenation early after birth (24,25), the earlier hypothermia can be installed the better its neuroprotective action may be! This is in fact also supported by earlier studies and passive or, better, active cooling (ice packs or servo-controlled) during transport to the NICU is therefore important (26-28). On the other hand, it has been suggested that delayed cooling after HIE, starting between 6 and 24 hours after birth, may have benefit as well as shown by Laptook et al. (29). HT is a relatively safe neuroprotective therapy although several (potential) complications are reported from which arrhythmia (mostly bradycardia) and thrombocytopenia are the most common ones (18,30,31).
Two additional practical questions remain: will an increase of the length of cooling time and/or the depth of core temperature further improve neurodevelopmental outcome? This was investigated in a randomized clinical trial in the US where they compared duration of cooling of 120 hours with the standard cooling time of 72 hours; both cooling groups were either cooled to 32.0 °C or to the standard temperature of 33.5 °C. The trial was discontinued because of futility or even possible harm, strongly suggesting that longer and/or deeper cooling was not beneficial indeed (32). Although both studies using selective head cooling or total body cooling showed significant improvements of outcome, whole body cooling may be superior (33,34).
Trials with HT and clinical practice nowadays limit the use of HT for term neonates who experienced birth asphyxia and developed a moderate to severe encephalopathy, mostly but not always defined using the Sarnat classification (35,36). A systemic review from 2018, including 20 studies, showed that infants with mild encephalopathy due to birth asphyxia had an adverse outcome in 25% of the infants, although the definition of “mild encephalopathy” used in these studies was not straight forward (36). However, Rao et al. reported recently that adverse outcome of infants with mild encephalopathy may be up to 43% (37). Moreover, van Handel et al investigated cognitive outcome at 9–10 years of age of a cohort of birth asphyxiated children with mild and moderate neonatal encephalopathy. They also found a substantial negative effect of mild neonatal encephalopathy on memory functioning in these children (38). This suggests that also neonates with a mild neonatal encephalopathy after birth asphyxia may benefit from HT. Another related issue is whether or not (moderate) preterm infants should be treated with HT: a retrospective study of 31 cooled preterm neonates born after a gestational age of 34–35 weeks compared to 31 term cooled neonates (37) showed that HT is feasible in this age group, but caution is warranted with respect to mortality and adverse effects (37,39,40).
At present, in our NICU, the inclusion criteria are a gestational age of 35.0 weeks or more, well documented perinatal asphyxia, followed by abnormal aEEG patterns (suppressed background patterns with a basline below 5 µV), or clinical criteria (Thompson score of 7 or more, or moderate to severe encephalopathy according to Sarnat). HT significantly decreased the composite outcome of mortality and adverse neurodevelopment in neonates with moderate (death/disability not cooled-cooled: 54% vs. 37%) and severe encephalopathy (death/disability not cooled-cooled: 86% vs. 70%) with a “number to treat” (NTT) of seven, meaning that one out of seven asphyxiated neonates treated with HT benefits from the intervention (18). However, with an overall composite adverse outcome (death; disabilities) of 40% to 50% negative consequences of birth asphyxia are still high (18,41) and it is conceivable that additional means of neuroprotection or recovery after birth asphyxia may further improve neurodevelopmental outcome. In this paper we will focus on pharmacological neuroprotection as add-on therapy for HT after severe perinatal hypoxia-ischemia (42).
Pharmacological add-on therapy for HT
To answer the question in what way pharmacological neuroprotection can further improve clinical outcome, a detailed knowledge of the time course of the already above mentioned destructive molecular pathways, set in motion after (severe) perinatal hypoxia-ischemia, is extremely important. When the time lines of (maximal) formation of (pro-) free radicals, cytokines and other toxic compounds are known optimal timing and optimal duration of antidotal treatment with specific drugs intervening in the formation of these destructive compounds can be determined.
Fetal therapy
In 90% of perinatal HI the starting point of birth asphyxia is fetal hypoxia leading to a failure of oxidative metabolism with consequent accumulation of hypoxanthine (8,43). Moreover, fetal hypoxia will trigger an excess formation of excitatory neurotransmitters, which activates the N-methyl-D-aspartate (NMDA) but also the voltage regulated receptors on the neuronal membrane (44). This results into a surge of extracellular calcium into the neurons and increase in intracellular Ca2+ leading to production of free radicals with damage of the membranes of neurons oligodendrocytes and astrocytes (24,44). This process is accompanied with a drop in intra and extra cellular pH. Liberation of metal-ions from the binding proteins leads to formation of so-called pro-radicals from which free or non protein-bound iron (Fe2+) is an important one (45,46). See also Figure 2.
Trials with maternal magnesium therapy during fetal asphyxia intending to reduce the fetal excitatory transmitter production by blocking the NMDA-receptors were not successful (47,48). Attempts to reduce fetal formation of free radicals with maternal therapy with vitamin C, phenobarbital, N-Acetyl cysteine or melatonin, all drugs were supposed to pass the placenta, did not show or suggest positive or conclusive results (49-52). Allopurinol, which is predominantly a xanthine oxidase inhibitor but in higher concentrations also a direct free radical scavenger and free ion chelator (53,54) will exert its optimal action in the very early postnatal period given the excessive surge of (O2∙) and free radicals occurring as early as 30–60 minutes after reoxygenation and reperfusion (25,55). Uploading the fetus with therapeutical levels of allopurinol by treating the mother diagnosed with fetal hypoxia optimizes the free radical reducing effects of allopurinol since therapeutical levels of allopurinol and its active metabolite oxypurinol can optimally neutralize free radical formation which immediately accelerates upon birth because of a renewed availability of oxygen. Earlier studies showed that oral and intravenous maternal treatment led to therapeutical levels of allopurinol and oxypurinol in the fetus within 20 minutes after the maternal administration (56,57). A double blinded randomized clinical trial in term pregnant women diagnosed with fetal hypoxia just before delivery showed a gender specific short-term positive effect in girls, but also a considerable overtreatment of pregnant women who did not deliver an asphyxiated baby (58). Long-term developmental outcome of this cohort did not reveal differences between allopurinol- and placebo treated groups. However, in this study almost all fetuses experienced mild to moderate hypoxia, not enough to eventually developing birth asphyxia (58,59).
Therapy in early neonatal period
After birth perfusion recovers normalizing oxygen availability. Since oxygen availability is very important for metabolization of the accumulated xanthine to uric acid by xanthine-oxidase during fetal hypoxia a huge production of O2∙ occurs upon reoxygenation with a maximal production already 30 to 60 minutes after birth. O2∙ Itself is destructive, but it also facilitates the metabolization of pro-radicals like free or non-protein bound iron, already produced fetally, generating the very aggressive hydroxyl free radical (OH∙). It further reacts with the nitric oxide free radical (NO∙) to form the very toxic peroxynitrite (ONOO−) (5,6,25,42,55). So, very early after birth excessive amounts of free radicals and other toxic products may damage the brain and to prevent the damaging effects of these compounds it is important to install free radical scavenging therapy as early as possible after birth. Although a “therapeutic window” of 6 hours following birth is generally accepted to be the standard and is considered to be the time “slot” to start HT, reducing or preventing free radical and nitric oxide formation as early as possible and preferably within 30 minutes after birth can be crucial to safe the newborn brain after birth asphyxia. As already stated above allopurinol may be an ideal candidate here (60). We showed that maternal allopurinol therapy to prevent excessive production of the superoxide free radical (O2;) is possible but led to a huge overtreatment of women diagnosed with fetal hypoxia (57-59). We therefore started a large European double blind randomized trial (ALBINO trial) where we aim to treat the neonates diagnosed with birth asphyxia within 45 minutes after birth with high dose allopurinol (or placebo) as add-on therapy of HT (ClinicalTrials.gov NCT03162653) (61). Clinical studies with allopurinol within the therapeutical window of 6 hours suggested a beneficial effect on short-term cerebral biomarkers and even on long-term developmental outcome, especially in moderately asphyxiated infants without serious adverse effects (62-64), but a Cochrane review in 2008 could not confirm that allopurinol was an effective neuroprotective therapy in the post hypoxic-ischemic neonatal brain since the clinical trials reported up to now were underpowered (65). The mostly relatively late treatment regimen in these clinical studies, up to 4 hours after the actual hypoxic-ischemic insult, may be an important cause for these ambiguous results.
As is the case with early allopurinol therapy, early inhibition of nitric oxide formation may be of value in relation with prevention of birth asphyxia-induced brain damage (66,67). Nitric oxide synthase (NOS) is an enzyme catalyzing production of NO∙ from L-arginine. Neuronal NOS (nNOS) and endothelial NOS (eNOS) are upregulated immediately after reperfusion/reoxygenation and inducible NOS (iNOS) from several hours onwards (68). eNOS is important in maintaining pulmonary blood flow, preventing pulmonary hypertension and maintaining adequate oxygenation. However, nNOS and iNOS are reported to have a damaging effect on the developing brain after perinatal asphyxia. Excessive NO∙ formation due to nNOS takes place especially in the early reperfusion/reoxygenation period shortly after birth, where it reacts with O2∙ to produce the toxic peroxynitrite and nitrotyrosine, an end product of this process (7). iNOS upregulation occurs somewhat later after birth and reach its maximal production at an age between 6 and 12 hours and has also been shown to augment brain injury (69). Therefore selective inhibition of nNOS and iNOS can be neuroprotective. This was confirmed in a recent review of experimental studies on NOS inhibition (66). Our research lab investigated in several animal models 2-Iminobiotin (2-IB), a selective nNOS and iNOS inhibitor, and confirmed its neuroprotective actions after hypoxia-ischemia (67,70,71). Recently, pharmacokinetics of 2-IB were investigated by us in human infants with perinatal asphyxia requiring therapeutic hypothermia (72). Also here it may be very important to start treatment immediately after birth, especially in respect with the inhibition of the nNOS formation. A large blinded randomized clinical trial to show the effectiveness of 2-IB is in preparation.
Xenon, a noble gas and antagonist of the NMDA receptor, preventing or reducing the influx of calcium into neuronal cells, has also strong anti-apoptotic properties (73,74). Xenon inhalation may therefore be a potential neuroprotective strategy and has been investigated as an add-on therapy for asphyxiated neonatal species (75-77). It shows a rapid passage of the blood-brain barrier, and has no negative side effects are reported. Add-on therapy with HT enhanced neuroprotection which is demonstrated in several species as compared to neuroprotection by HT alone (78). Xenon use is costly, needs a closed loop system with adjustments in neonatal ventilators and cuffed tracheal tubes to stop loss of Xenon (79). The only clinical study using Xenon as add-on therapy for HT showed no adverse effects, but also no additional neuroprotective effect of Xenon (80). Ventilation with Argon may be an alternative: Argon is another noble gas with reported neuroprotective actions in experimental studies in the neonatal animal, is much cheaper and does not need a closed loop system (81,82).
Magnesium has been shown to decrease hypoxic-ischemic excitotoxic activity by closing the NMDA glutamate channel by binding to the magnesium site and thus stabilizing the neuronal cell membrane (47). The neuroprotective action of Magnesium in term infants has been tested in several studies, but a meta-analysis showed no differences in mortality (83-85). At present there is no evidence that MgSO4 as add-on therapy for hypothermia is neuroprotective after perinatal asphyxia (86,87).
Melatonin exerts a neuroprotective action after perinatal asphyxia by reduction of oxidative stress. It should have also anti-inflammatory, and anti-apoptotic activities (88). A neonatal piglet model of perinatal hypoxia-ischemia reported significant neuroprotection (89), and 2 pilot studies, from which one used melatonin as add-on therapy during HT, reported short-term neuroprotective effects as indicated by less free radical and NO∙ formation, and neurological improvement compared to hypothermia as single therapy respectively (90,91). Confirmation of its neuroprotective effects after birth asphyxia in the clinical situation is pending.
N-acetylcysteine, glutathione precursor, exerts a direct free radical scavenging activity (92). Although experimental studies revealed neuroprotective actions as add-on therapy for HT (93), adverse reactions were reported (78). Clinical studies in asphyxiated newborns are scarce and non-conclusive (94).
Some other drugs are mentioned in relation with neuroprotection after perinatal hypoxia-ischemia mostly because of their free radical scavenging effect such as Tetrahydrobiopterin and Topiramate, but their clinical significance is not yet well defined (78,95).
Finally, hyperbaric oxygen therapy should be mentioned in relation with its antioxidative actions: supranormal concentrations of oxygen aim to induce tissue super oxide dismutase expression and thus production and by that enhancing the antioxidative capacity of the body. Serious studies are needed to bring this antioxidative approach to the clinic (96).
As already stated above, a start as early as possible after birth of free radical scavenging therapy after perinatal asphyxia are expected to enhance the neuroprotective effects. Figure 2 summarizes the timelines of production of free (pro) radicals and other toxic compounds and shows the preferred therapeutic actions of the most promising drugs as a function of postnatal age.
Delayed therapy (>6 hours of age); enhancement of anti-inflammation and neurotrophic factors
The transcription factor nuclear factor kappa B (NFB), which regulates expression of genes involved in inflammation and apoptosis, is upregulated after perinatal hypoxia-ischemia (97). This activation of NFB is partly due to and follows on the surge of free radicals upon and early after reperfusion and reoxygenation and manifests itself about 6 to 12 hours after birth. This potentially destructive pathway leads to an abundant formation of pro- and anti-inflammatory cytokines, starts production of inducible NOS (iNOS) and induces inappropriate apoptosis (see also Figure 2). At this particular postnatal time frame secondary energy failure of brain metabolism and subsequent brain damage becomes manifest. This brain damage is increasingly linked to pro-inflammation and excessive apoptosis due to lack of neurotropic repair activity (98).
Boosting anti-inflammatory capacity and repair by neurotropic factors seems essential. Several drugs and compounds are promising and most well investigated in this respect is probably Erythropoietin (EPO). EPO and EPO-derivatives (asialo-EPO and darbepoetin) have anti-inflammatory, anti-apoptotic and neurotropic properties as reported in experimental studies (99-101). EPO and its derivates exert its neuroprotective action in neurons, oligodendrocytes and astrocytes via hypoxia-mediated activation of the transcription factor hypoxia-inducible factor-1a (99). Recombinant Human EPO (rhEPO) has a safe pharmacological profile and is already used in preterm infants (102). Clinically a small trial suggested that therapy in asphyxiated newborns with rhEPO appeared safe and feasible (103). In China, a double-blinded randomized trial, before the HT era, in perinatally asphyxiated neonates showed that intravenously administered rhEPO improved developmental outcome at 18 months of age. Dosages used in this study where quite low (750 IU/kg) and there appeared a gender preference for girls who benefited most from this therapy (104). A pharmacokinetic study in 24 neonates with asphyxia undergoing HT and treated with rhEPO as add-on therapy for HT, showed that repeated high dose rhEPO (1,000 U/kg) were well tolerated (105). Randomized trials with moderate hypothermia and add-on therapy with high dose rhEPO was safe and feasible and suggested to reduce MRI-derived brain injury and improve 1-year motor function (106,107). A recent meta-analysis of 6 randomized trials with rhEPO or EPO-derivatives as add-on therapy for HT reported a lower risk for brain damage and cognitive outcome (108). Optimally powered studies are needed with appropriate (high) dosages of rhEPO and/or EPO-derivatives to ultimately confirm neuroprotective properties of this drug. A phase III double blind randomized study is currently underway (the HEAL Trial; ClinicalTrials.gov NCT02511263).
Melatonin has also anti-inflammatory properties, especially by preventing activation and translocation of NFB to the nucleus, in addition to its anti-oxidative and free radical scavenging actions (88). Although melatonin is a promising drug with respect to neuroprotection after perinatal hypoxia-ischemia, no clinical studies have been reported with respect to its anti-inflammatory action.
At present, two promising compounds, only investigated in the experimental setting at this point, should be mentioned: cannabinoids and azithromycin. Cannabinoids has anti-neurotoxic and anti-inflammatory properties and exert brain protection after neonatal hypoxia-ischemia as has been reported in the experimental setting, also as add-on therapy (98,109). Azithromycin is an antibiotic and a macrolide derivative of erythromycin (110). Azithromycin improved functional and neuropathology outcomes, probably based on its anti-inflammatory properties (111).
Research with respect to inhibition of NFB and pro-inflammatory cytokines or enhancement of anti-inflammatory cytokines or anti-apoptotic strategies are very interesting but still preliminary (112).
Downregulation of neurotropic-, maturational- and growth factors in the first days and even weeks after severe perinatal hypoxia-ischemia with an increased apoptotic activity has a negative influence on developmental outcome (99). It has been reported that erythropoietin has neurotropic and anti-apoptotic properties (113,114). EPO has a long lasting positive effect on neurogenesis but also on neovascularization, which is in particular true if given in supranormal dosages (1,000 to 5,000 IU/kg) improving developmental outcome. Sustained therapy may be important here, but need more research in relation with drug safety (115). HIF-1α-induced extra formation of transcriptional targets, enhanced formation of erythropoietin, endothelial growth factor and consequent brain repair may be a very important mechanistic issue here (100).
An intriguing recent field of research in respect with prevention and repair of immature brain damage is boosting and/or elevation of (endogenous) neurotropic factors such as basic fibroblast growth factor (BFGF), BDNF and especially IGF-1. Animal studies showed a reduction of injury of the (preterm) neonatal brain (116,117). Future research may demonstrate whether this therapy contributes to an improved long-term neurodevelopmental outcome after severe perinatal hypoxia-ischemia.
Repair of the brain after birth asphyxia-derived brain damage
Embryonic stem cells have the potential to differentiate into hematopoietic, neural or mesenchymal stem cells (118,119). Among the 3 lineages of the embryonic stem cell the mesenchymal stem cell (MSC) is the stem cell of choice at present for clinical studies and treatment options because of its neuro-regenerative and important immune-modulating actions and proven safety (120,121). Contrary to embryonic or neural stem cells, which are linked to the induction of tumorous formation, MSCs have a well investigated safety profile (121). As a detailed discussion of the potential benefits and hazards of several types of stem cells is beyond the scope of this review, we refer to a recent review paper we published about the therapeutic value of MSCs as compared to other progenitor cells (118).
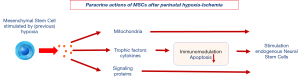
Besides it safety profile the strength of MSCs in clinical care lies predominantly in the fact that MSCs can, in addition to differentiating into mesodermal cells (fat, bone and cartilage), induce proliferation of endogenous neural stem cells if entering a (previous) hypoxic environment (122,123): MSCs stimulate this formation of new brain cells by a paracrine action rather than by transformation themselves into neuronal tissue (118,124). See also Figure 3. A more detailed discussion of the beneficial actions of MSCs after neonatal brain injury are well-described in a recent review paper of our stem cell research group by Wagenaar et al. (118).
Optimal results by combining therapies at the right point of time
The ultimate answer towards a further reduction of brain damage after perinatal asphyxia by pharmacological neuroprotection is not yet available. However, if calcium influx can be reduced, free (pro-) radical production and toxic compounds in the fetal and early post-ictal phase can be reduced or even prevented. Subsequently, the inflammatory activation and loss of trophic factors can be restrained which requires besides HT multiple pharmacological interventions requiring optimal timing, dosing and duration for each drug dedicated to its specific intervention in the (destructive) molecular pathway. The major challenge for pharmacologic therapy after perinatal hypoxia-ischemia is to figure out the optimal combination of pharmaceuticals as add-on therapy with HT. Unconventional study set-ups are necessary to prove the clinical value of such a pharmacologic approach! Additional issues such as gender specificity of the drugs used have to be resolved too. But if we are able to solve the complex questions as stated above and can “repair” remaining brain injury with cell therapy, the long-term outcome of neonates who experienced severe perinatal asphyxia can be improved substantially!
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.11.02). Herewith the authors of this manuscript, Frank van Bel, MD and Floris Groenendaal, MD declare that Frank van Bel and Floris Groenendaal are, together with Cacha Peeters-Scholte, inventors of 2-iminobiotin as neuroprotective agent for neonates with cerebral hypoxia-ischemia. They have no financial or personal relationships with other people or organizations that could potentially and inappropriately influence their work and conclusions. They further declare to have no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martinello K, Hart AR, Yap S, et al. Management and investigation of neonatal encephalopathy; 2017 update. Arch Dis Childh Fetal Neonatal Ed 2017;102:F346-58.
- Aminu M, Unkels R, Mdegela M, et al. Causes of and factors associated with stillbirth in low- and middle-income countries: a systematic literature review. BJOG 2014;121:141-53. [Crossref] [PubMed]
- Badawi N, Felix JF, Kurinczuk JJ, et al. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol 2005;47:293-8. [Crossref] [PubMed]
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430-40. [Crossref] [PubMed]
- Saugstad OD, Oei JL, Lakshminrusimha S, et al. Oxygen therapy of the newborn from molecular understanding to clinical practice. Pediatr Res 2019;85:20-29. [Crossref] [PubMed]
- Qin X, Cheng J, Zhong Y, et al. Mechanism and Treatment Related to Oxidative Stress in Neonatal Hypoxic-Ischemic Encephalopathy. Front Mol Neurosci 2019;12:88. [Crossref] [PubMed]
- Groenendaal F, Lammers H, Smit D, et al. Nitrotyrosine in brain tissue of neonates after perinatal asphyxia. Arch dis Child Fetal Neonatal Ed. 2006;91:F429-33. [Crossref] [PubMed]
- Glass HC, Ferriero DM. Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol 2007;9:414-23. [Crossref] [PubMed]
- Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr 2005;146:453-60. [Crossref] [PubMed]
- de Vries LS, Groenendaal F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology 2010;52:555-66. [Crossref] [PubMed]
- Penrice J, Lorek A, Cady EB, et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr Res 1997;41:795-802. [Crossref] [PubMed]
- Gunn AJ, Laptook AR, Robertson NJ, et al. Therapeutic hypothermia translates from ancient history in to practice. Pediatr Res 2017;81:202-209. [Crossref] [PubMed]
- Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol 2012;11:556-66. [Crossref] [PubMed]
- Gunn AJ, Gunn TR, Gunning MI, et al. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 1998;102:1098-106. [Crossref] [PubMed]
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574-84. [Crossref] [PubMed]
- Hobbs C, Thoresen M, Tucker A, et al. Xenon and hypothermia combine additively, offering long-term functional and histo-pathologic neuroprotection after neonatal hypoxia/ischemia. Stroke 2008;39:1307-13. [Crossref] [PubMed]
- Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild system-hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in china. J Pediatr 2010;157:367-372, 72e-3.
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;1:CD003311 [PubMed]
- Shankaran S, Pappas A, McDonalds SA, et al. Childhood outcomes after hypothermia fro neonatal encephalopathy. N Engl. J Med 2012;366:2085-92. [Crossref] [PubMed]
- Azzopardi D, Strohm B, Marlow N, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 2014;371:140-9. [Crossref] [PubMed]
- Bona E, Hagberg H, Loberg EM, et al. Protective effects of moderate hypothermia after neonatal hypoxia-ischenia: short – and long-term outcome. Pediatr Res 1998;43:738-45. [Crossref] [PubMed]
- Gunn AJ, Gunn TR, de Haan HH, et al. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 1997;99:248-56. [Crossref] [PubMed]
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997;8:3359-62. [Crossref] [PubMed]
- Ferriero DM. Neonatal brain injury. N Engl J Med 2004;351:1985-95. [Crossref] [PubMed]
- Dirnagl U, Lindauer U, Them A, et al. Global cerebral ischemia in the rat: online monitoring of oxygen free radical production using chemiluminescence in vivo. J Cereb Blood Flow Metab 1995;15:929-40. [Crossref] [PubMed]
- Thoresen M, Tooley J, Liu X, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 2013;104:228-33. [Crossref] [PubMed]
- O’Reilly KM, Tooley J, Winterbottom S. Therapeutic hypothermia during neonatal transport. Acta Paediatr 2011;100:1084-6. [Crossref] [PubMed]
- Gunn J, Groenendaal F. Delayed neuroprotection in the era of hypothermia: what can be add? J Clin Neonatol 2016;5:3-7. [Crossref]
- Laptook AR, Shankaran S, Tyson JE, et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy. A randomized clinical trial. JAMA 2017;318:1550-60. [Crossref] [PubMed]
- Shah PS. Hypothermia: a systemic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med 2010;15:238-46. [Crossref] [PubMed]
- Diederen CMJ, van Bel F, Groenendaal F. Complications during therapeutic hypothermia after perinatal asphyxia: A comparison with trial data. Ther Hypothermia Temp Manag 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: A randomized clinical trial. JAMA 2014;312:2629-39. [Crossref] [PubMed]
- Sarkas S, Donn SM, Bapuraj JR, et al. Distribution and severity of hypoxic-ischaemic lesions on brain MRI following therapeutic cooling: selective head vs whole body cooling. Arch Dis Child Fetal Neonatal Ed 2012;97:335-9. [Crossref]
- Goenka A, Yozawitz E, Gomes WA, et al. Selective head versus whole body cooling treatement of hypoxic-ischemic encephalopathy: comparison of electroencephalogram and magnetic resonance imaging findings. Am J Perinatol 2019; [Epub ahead of print]. [PubMed]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696-705. [Crossref] [PubMed]
- Conway JM, Walsh BH, Boylan GB, et al. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome-A systematic review. Early Hum Dev 2018;120:80-7. [Crossref] [PubMed]
- Rao R, Trivedi S, Vesoulis Z, et al. Safety and short-term outcomes of therapeutic hypothermia in preterm neonates 34-35 weeks gestational age with hypoxic-ischemic encephalopathy. J Pediatr 2017;183:37-42. [Crossref] [PubMed]
- van Handel M, de Sonneville L, de Vries LS, et al. Specific memory impairment following neonatal encephalopathy in term-born children. Dev Neuropsychol 2012;37:30-50. [Crossref] [PubMed]
- Walsh W, Butler D, Schmidt JW. Report of a pilot study of cooling four preterm infants 32-35 weeks gestation with HIE. J Neonatal Perinatal Med 2015;8:47-51. [Crossref] [PubMed]
- Bennet L, Roelfsema V, George S, et al. The effect of cerebral hypothermia on white and grey matter injury induced by evere hypoxia in preterm fetal sheep. J Physiol 2007;578:491-506. [Crossref] [PubMed]
- Shankaran S. Outcomes of hypoxic-ischemic encephalopathy in neonates treated with hypothermia. Clin Perinatol 2014;41:149-59. [Crossref] [PubMed]
- Bel F, Groenendaal F. Drugs for neuroproetection after birth asphyxia: Pharmacologic adjuncts to hypothermia. Semin Perinatol 2016;40:152-9. [Crossref] [PubMed]
- Saugstad OD. Role of xanthine-oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics 1996;98:103-7. [PubMed]
- Hilton GD, Nunez JL, Bambrick L, et al. MGlutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGlur-induced release of CA++ from intracellular stores and is prevented by estradiol. Eur J Neurosci 2006;24:3008-16. [Crossref] [PubMed]
- van Bel F, Dorrepaal CA, Benders MJ, et al. Changes in cerebral hemodynamics and oxygenation in the first 24 hours after birth asphyxia. Pediatrics 1993;92:365-72. [PubMed]
- Shadid M, Buonocore G, Groenendaal F, et al. Effect of deferoxamine and allopurinol on non protein-bound iron concentrations in plasma and cortical brain tissue of newborn lambs following hypoxia-ischemia. Neurosci let 1998;22:5-8.
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 1994;330:613-22. [Crossref] [PubMed]
- Nguyen TM, Crowther CA, Wilkinson D, et al. Magnesium sulphate for women at term for neuroprotection of the fetus. Cochrane Database Syst Rev 2013;CD009395 [PubMed]
- Vásquez-Vivar J, Whitsett J, Derrick M, et al. Tetrahydrobiopterin in the prevention of hypertonia in hypoxic fetal brain. Ann Neurol 2009;66:323-31. [Crossref] [PubMed]
- Reinisch JM, Sanders SA, Mortensen EL, et al. In utero exposure to phenobarbital and intelligence deficits in adult men. JAMA 1995;274:1518-25. [Crossref] [PubMed]
- Xu DX, Chen YH, Wang H, et al. Effect of N-acetylcysteine on lipopolysaccharide-induced intra-uterine fetal death and intra-uterine growth retardation in mice. Toxicol Sci 2005;88:525-33. [Crossref] [PubMed]
- Jahnke G, Marr M, Myers C, et al. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol Sci 1999;50:271-9. [Crossref] [PubMed]
- Ko KM, Godin DV. Inhibition of transition metal ion-catalysed ascorbate oxidation and lipid peroxidation by allopurinol and oxypurinol. Biochem Pharmacol 1990;40:803-9. [Crossref] [PubMed]
- Moorhouse PC, Grootveld M, Halliwell B, et al. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett 1987;213:23-8. [Crossref] [PubMed]
- Ono T, Tsuruta R, Fujita M, et al. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Res 2009;1305:158-67. [Crossref] [PubMed]
- Boda D, Nemeth I, Kiss P, et al. Treatment of mothers with allopurinol to produce therapeutic blood levels in newborns. Prenatal and Neonatal Medicine 1999;4:130-4.
- Torrance HL, Benders MJ, Derks JB, et al. Maternal allopurinol treatment during fetal hypoxia lowers cord blood levels of the brain injury marker protein S-100B. Pediatrics 2009;124:350-7. [Crossref] [PubMed]
- Kaandorp JJ, Benders MJ, Schuit E, et al. Maternal allopurinol administration during suspected fetal hypoxia: a novel neuroprotective intervention? A multicentre randomised placebo controlled trial. Arch Dis Child Fetal Neonatal Ed 2015;100:F216-23. [Crossref] [PubMed]
- Klumper J, Kaandorp JJ, Schuit E, et al. Behavioral and neurodevelopmental outcome of children after maternal allopurinol administration during suspected fetal hypoxia: 5-year follow up of the ALLO-trial. PLoS One 2018;13:e0201063 [Crossref] [PubMed]
- Annink KV, Franz AR, Derks JB, et al. Allopurinol: old drug, new indications in neonates? Curr Pharm Des 2017;23:5935-42. [Crossref] [PubMed]
- Maiwald CA, Annink KV, Rudiger M, et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (ALBINO): study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III). BMC Pediatr 2019;19:210. [Crossref] [PubMed]
- Van Bel F, Shadid M, Moison RMW, et al. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics 1998;101:185-93. [Crossref] [PubMed]
- Gunes T, Ozturk MA, Koklu E, et al. Effect of allopurinol supplementation on nitric oxide levels in asphyxiated newborns. Pediatr Neurol 2007;36:17-24. [Crossref] [PubMed]
- Kaandorp JJ, van Bel F, Veen S, et al. Long-term neuroprotective effects of allopurinol after moderate perinatal asphyxia: follow-up of two randomised controlled trials. Arch Dis Child Fetal Neonatal Ed 2012;97:F162-6. [Crossref] [PubMed]
- Chaudhari T, McGuire W. Allopurinol for preventing mortality and morbidity in newborn infants with suspected hypoxic-ischaemic encephalopathy. Cochrane Database Syst Rev 2008;CD006817 [PubMed]
- Favié LMA, Cox AR, van de Hoogen A, et al. Nitric oxide synthase inhibition as a neuroprotective strategy following hypoxic-ischemic encephalopathy: evidence from animal studies. Front Neurol 2018;9:258. [Crossref] [PubMed]
- Peeters-Scholte C, Koster J, Veldhuis W, et al. Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hyopoxia-ischemia. Stroke 2002;33:2304-10. [Crossref] [PubMed]
- Liu H, Li J, Zhao F, et al. Nitric oxide synthase in hypoxic or ischemic brain injury. Rev Neurosci 2015;26:105-17. [Crossref] [PubMed]
- Brown GC, Vilalta A. How microglia kill neurons. Brain Res 2015;1628:288-97. [Crossref] [PubMed]
- Nijboer CH, Kavelaars A, van Bel F, et al. Gender-dependent pathways of hypoxia-ischemia-induced cell death and neuroprotection in the immature P3 rat. Dev Neurosci 2007;29:385-92. [Crossref] [PubMed]
- Zitta K, Peeters-Scholte C, Sommer L, et al. 2-Iminobiotin Superimposed on Hypothermia Protects Human Neuronal Cells from Hypoxia-Induced Cell Damage: An in Vitro Study. Front Pharmacol 2018;8:971. [Crossref] [PubMed]
- Favié LMA, Peeters-Scholte CMPCD, Bakker A, et al. Pharmacokinetics and short term safety of the selective NOS inhibitor 2-imiobiotin in asphyxiated neonates treated with therapeutic hypothermia. Pediatr Res 2020;87:689-96. [PubMed]
- Ma D, Williamson P, Januszewski A, et al. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology 2007;106:746-53. [Crossref] [PubMed]
- Ma D, Lim T, Xu J, et al. Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1alpha activation. J Am Soc Nephrol 2009;20:713-20. [Crossref] [PubMed]
- Thoresen M, Hobbs CE, Wood T, et al. Cooling combined with immediate or delayed xenon inhalation provides equivalent long-term neuroprotection after neonatal hypoxia-ischemia. J Cereb Blood Flow Metab 2009;29:707-14. [Crossref] [PubMed]
- Faulkner S, Bainbridge A, Kato T, et al. Xenon augmented hypothermia reduces early lactate/N-acetylaspartate and cell death in perinatal asphyxia. Ann Neurol 2011;70:133-50. [Crossref] [PubMed]
- Liu X, Dingley J, Scull-Brown E, et al. Adding 5 h delayed xenon to delayed hypothermia treatment improves long-term function in neonatal rats surviving to adulthood. Pediatr Res 2015;77:779-83. [Crossref] [PubMed]
- Robertson NJ, Tan S, Groenendaal F, et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatr 2012;160:544-52.e4. [Crossref] [PubMed]
- Chakkarapani E, Thoresen M, Hobbs CE, et al. A closed-circuit neonatal xenon delivery system: a technical and practical neuroprotection feasibility study in newborn pigs. Anesth Analg 2009;109:451-60. [Crossref] [PubMed]
- Azzopardi D, Robertson NJ, Bainbridge A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol 2016;15:145-53. [Crossref] [PubMed]
- Zhuang L, Yang T, Zhao H, et al. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit Care Med 2012;40:1724-30. [Crossref] [PubMed]
- Alderliesten T, Favie LM, Neijzen RW, et al. Neuroprotection by argon ventilation after perinatal asphyxia: a safety study in newborn piglets. PLoS One 2014;9:e113575 [Crossref] [PubMed]
- Tagin M, Shah PS, Lee KS. Magnesium for newborns with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J Perinatol 2013;33:663-9. [Crossref] [PubMed]
- Groenendaal F, Rademaker CMA, Toet MC, et al. Effects of magnesium sulfate on amplitude-integrated continuous EEG in asphyxiated term neonates. Acta Paediatrica 2002;91:1073-7. [Crossref] [PubMed]
- Bhat MA, Charoo BA, Bhat JI, et al. Magnesium sulfate in severe perinatal asphyxia: a randomized, placebo-controlled trial. Pediatrics 2009;123:e764-9. [Crossref] [PubMed]
- Rahman SU, Canpolat FE, Oncel MY, et al. Multicenter Randomized Controlled Trial of Therapeutic Hypothermia Plus Magnesium Sulfate Versus Therapeutic Hypothermia Plus Placebo in the Management of Term and Near - term Infants with Hypoxic Ischemic Encephalopathy (The Mag Cool Study): A Pilot Study. J Clin Neonatol 2015;4:158-63. [Crossref]
- Galinsky R, Bennet L, Groenendaal F, et al. Magnesium is not consistently neuroprotective for perinatal hypoxia-ischemia in term-equivalent models in preclinical studies: a systematic review. Dev Neurosci 2014;36:73-82. [Crossref] [PubMed]
- Colella M, Biran V, Baud O. Melatonin and the newborn brain. Early Hum Dev 2016;102:1-3. [Crossref] [PubMed]
- Robertson NJ, Faulkner S, Fleiss B, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2013;136:90-105. [Crossref] [PubMed]
- Fulia F, Gitto E, Cuzzocrea S, et al. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: reduction by melatonin. J Pineal Res 2001;31:343-9. [Crossref] [PubMed]
- Aly H, Elmahdy H, El-Dib M, et al. Melatonin use for neuroprotection in perinatal asphyxia: a randomized controlled pilot study. J Perinatol 2015;35:186-91. [Crossref] [PubMed]
- Aruoma OI, Halliwell B, Hoey BM, et al. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 1989;6:593-7. [Crossref] [PubMed]
- Jatana M, Singh I, Singh AK, et al. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res 2006;59:684-9. [Crossref] [PubMed]
- Jenkins DD, Wiest DB, Mulvihill DM, et al. Fetal and Neonatal Effects of N-Acetylcysteine When Used for Neuroprotection in Maternal Chorioamnionitis. J Pediatr 2016;168:67-76.e6. [Crossref] [PubMed]
- Marques MR, Garcia-Robles A, Usach I, et al. Topiramate pharmacokinetics in neonates underging therapeutic hypothermia and proposal of an optimised dosing schedule. Acta Paediatr 2020;109:300-8. [PubMed]
- Liu S, Shen G, Deng S, et al. Hyperbaric oxygen therapy improves cognitive functioning after brain injury Neural Regen Res 2013;8:3334-43. [PubMed]
- Nijboer CH, Heijnen CJ, Groenendaal F, et al. A Dual Role of the NF-{kappa}B Pathway in Neonatal Hypoxic-Ischemic Brain Damage. Stroke 2008;39:2578-86. [Crossref] [PubMed]
- Hassell KJ, Ezzati M, Alonso-Alconada D, et al. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal Ed 2015;100:F541-52. [Crossref] [PubMed]
- Alagappan D, Lazzarino DA, Felling RJ, et al. Brain injury expands the numbers of neural stem cells and progenitors in the SVZ by enhancing their responsiveness to EGF. ASN Neuro 2009;1: [Crossref] [PubMed]
- Fan X, Heijnen CJ, van der Kooij MA, et al. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev 2009;62:99-108. [Crossref] [PubMed]
- van der Kooij MA, Groenendaal F, Kavelaars A, et al. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev 2008;59:22-33. [Crossref] [PubMed]
- Dame C, Juul SE, Christensen RD. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol Neonate 2001;79:228-35. [Crossref] [PubMed]
- Elmahdy H, El-Mashad AR, El-Bahrawy H, et al. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics 2010;125:e1135-42. [Crossref] [PubMed]
- Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics 2009;124:e218-26. [Crossref] [PubMed]
- Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics 2012;130:683-91. [Crossref] [PubMed]
- Frymoyer A, Juul SE, Massaro AN, et al. High-dose erythropoietin population pharmacokinetics in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. Pediatr Res 2017;81:865-72. [Crossref] [PubMed]
- Wu YW, Mathur AM, Chang T, et al. High-Dose Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy: A Phase II Trial. Pediatrics 2016;137: [Crossref] [PubMed]
- Razak A, Hussain A. Erythropoietin in perinatal hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J Perinat Med 2019;47:478-89. [Crossref] [PubMed]
- Pazos MR, Mohammed N, Lafuente H, et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology 2013;71:282-91. [Crossref] [PubMed]
- Smith C, Egunsola O, Choonara I, et al. Use and safety of azithromycin in neonates: a systematic review. BMJ Open 2015;5:e008194 [Crossref] [PubMed]
- Barks JDE, Liu Y, Wang L, et al. Repurposing azithromycin for neonatal neuroprotection. Pediatr Res 2019;86:444-51. [Crossref] [PubMed]
- Nijboer CH, Heijnen CJ, Groenendaal F, et al. Strong Neuroprotection by Inhibition of NF-{kappa}B After Neonatal Hypoxia-Ischemia Involves Apoptotic Mechanisms but Is Independent of Cytokines. Stroke 2008;39:2129-37. [Crossref] [PubMed]
- Campana WM, Misasi R, O'Brien JS. Identification of a neurotrophic sequence in erythropoietin. Int J Mol Med 1998;1:235-41. [PubMed]
- Iwai M, Stetler RA, Xing J, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke 2010;41:1032-7. [Crossref] [PubMed]
- Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 2009;40:e647-56. [Crossref] [PubMed]
- Lin S, Fan LW, Rhodes PG, et al. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Exp Neurol 2009;217:361-70. [Crossref] [PubMed]
- Galvin KA, Oorschot DE. Continuous low-dose treatment with brain-derived neurotrophic factor or neurotrophin-3 protects striatal medium spiny neurons from mild neonatal hypoxia/ischemia: a stereological study. Neuroscience 2003;118:1023-32. [Crossref] [PubMed]
- Wagenaar N, Nijboer CH, van Bel F. Repair of neonatal brain injury: bringing stem cell-based therapy into clinical practice. Dev Med Child Neurol 2017;59:997-1003. [Crossref] [PubMed]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 2000;6:88-95. [Crossref] [PubMed]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726-36. [Crossref] [PubMed]
- Fleiss B, Guillot PV, Titomanlio L, et al. Stem cell therapy for neonatal brain injury. Clin Perinatol 2014;41:133-48. [Crossref] [PubMed]
- Dezawa M, Kanno H, Hoshino M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest 2004;113:1701-10. [Crossref] [PubMed]
- van Velthoven CT, Kavelaars A, van Bel F, et al. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun 2010;24:387-93. [Crossref] [PubMed]
- van Velthoven CT, Kavelaars A, van Bel F, et al. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav Immun 2011;25:1342-8. [Crossref] [PubMed]
Cite this article as: van Bel F, Groenendaal F. Birth asphyxia-induced brain damage: the long road to optimal reduction and prevention! Pediatr Med 2020;3:3.

