Amenorrhea in adolescents: a narrative review
Introduction
While menstrual disorders can be common amongst adolescents, particularly in the first few years after menarche, amenorrhea warrants deliberate evaluation and potential treatment. Amenorrhea is defined as the absence or abnormal cessation of menstruation in women of reproductive age and can be divided into primary and secondary amenorrhea (1,2). The causes of primary and secondary amenorrhea are quite vast, with significant overlap between the two categories. Hence, a better way of classifying etiologies is analyzing the regulatory processes that occur within the hypothalamic-pituitary-ovarian (HPO) axis and the outflow tract. In determining the principal etiologies of a patient’s amenorrhea, a thorough history and physical exam with particular attention to pubertal development become of incredible importance. Understanding where particularly in the menstrual cycle or the affected regulatory process amenorrhea is occurring should lead to more effective treatment specific to the patient’s condition (2). Early detection in adolescent females help prevent potential future adverse health outcomes. This article provides an overview of the different causes, evaluation and treatment approach of amenorrhea, with special focus on polycystic ovary syndrome (PCOS), eating disorders (EDs), and the female athlete triad (FAT).
Methods
A literature search was performed on the electronic databases including MEDLINE and PubMed, from 1990 to 2019, to identify all relevant studies and reviews. A combination of the search terms included “menstrual, primary amenorrhea, secondary amenorrhea, athlete, polycystic ovary, eating disorder, and adolescent”. The reference list also included studies identified manually, and studies referenced for other purposes.
Menstrual physiology
The presence of an intact HPO axis is key in determining normal progression of development. Menarche is a normal occurring phenomenon in all pubertal girls with its usual timing occurring at least two years from thelarche. In the United States, the average age of menarche for Caucasian female adolescent in 12.6 years compared to 12.0 years in their African American peers. Several genetic and environmental factors affect the initiation and progression in otherwise healthy female adolescents (3).
The menstrual cycle occurs in a synchronized pattern of expected events as dictated by the HPO axis activity and is divided into the follicular (proliferative) and the luteal (secretory) phases. During normal ovulation, the gonadotropin-releasing hormone (GnRH) acts on the anterior pituitary gland releasing the follicle-stimulating hormone (FSH). Increase in FSH levels results in recruitment and eventual maturation of an ovarian follicle destined to undergo ovulation during the next menstrual cycle. Following the luteinizing hormone (LH) surge mid-cycle, the chosen follicle will be released. Release of estrogen from the follicle supports endometrial proliferation, and release of progesterone from the corpus luteum stabilizes it and promotes the endometrial secretory glands. If 14 days post-ovulation no fertilization of the ovum occurs, there is rapid decline of the hormonal levels followed by endometrial regression signaling the next menstrual cycle to begin (3).
Primary and secondary amenorrhea
Failure to attain menarche is primary amenorrhea. Evaluation is needed if menarche has not occurred by: age 15 years in females with normal secondary sexual development; age 13 years in females lacking any secondary sexual characteristics; within five years after thelarche. On the other hand, secondary amenorrhea is defined as menstrual cessation for at least three months in those with previously established regular menses, or lack of menses for over six months in patients who previously experienced irregular menses (1-4). Table 1 shows the different etiologies of amenorrhea as subdivided into primary and secondary causes with occasional overlapping between the two.
Table 1
| Primary amenorrhea | Secondary amenorrhea |
|---|---|
| Genetic abnormalities | Pregnancy |
| Turner syndrome | Breastfeeding |
| Gonadal dysgenesis | Hypothalamic |
| Kallmann syndrome | Functional hypothalamic amenorrhea |
| Anatomic | Tumors of the hypothalamus |
| Imperforate hymen | Pituitary |
| Transverse vaginal septum | Hyperprolactinemia |
| Mayer-Rokitansy-Kuster-Hauser Syndrome | Sheehan syndrome |
| Hypothalamic/pituitary | Radiation |
| Functional hypothalamic amenorrhea | Pituitary gland lesions |
| Idiopathic hypogonadotropic hypogonadism | Uterine |
| Delayed adrenarche and gonadarche | Asherman syndrome |
| Constitutional delay | Ovarian |
| Ovarian | Premature ovarian failure |
| Primary ovarian insufficiency | Polycystic ovary syndrome |
| Autoimmune oophoritis | Systemic |
| Polycystic ovary syndrome | Chronic diseases |
| Systemic diseases | Type 1 diabetes mellitus |
| Craniopharyngioma | Celiac disease |
| Germinoma | Thyroid |
| Langerhans cell histiocytosis | Hypothyroidism (more common) |
| Sellar masses | Hyperthyroidism |
| Deficiencies | Adrenal disease |
| Androgen insensitivity syndrome | Tumors |
| 5-alpha reductase deficiency | Medications |
| 17-alpha hydroxylase deficiency | Psychotropics |
| Contraceptives |
Another classification of identifying causes of amenorrhea is understanding the regulatory processes that occur at the HPO axis and outflow tract. Any disruption of the normal signaling within the axis may manifest as primary or secondary amenorrhea. At the hypothalamic level, the GnRH is responsible in the hormonal cascade events required for the onset of puberty. Thelarche is the first sign of puberty. Presence of breast development means the HPO axis has been activated. Females with constitutional delay of puberty usually show signs of breast development later than their peers. Hormonal milieu will reveal a low to low to normal gonadotropin levels in an otherwise healthy adolescent with a normal workup. The bone age is expected to be delayed compared to the chronological age (1).
The pituitary releases FSH and LH once it receives the right signal from the hypothalamus. The usual causes at the pituitary level can be attributed to increased prolactin secretion that can be central or peripheral in cause. It is important to rule out an occupying pituitary lesion in females with amenorrhea, headaches, and vision changes. At the ovarian level, low reserve before or after puberty results in amenorrhea. Significantly high FSH levels are detected in the setting of low or almost absent estradiol levels. In those with obvious gonadal failure, like in Turner patients, there is expected future challenges in fertility and bone health. Although rare, amenorrhea can be secondary to hypoplastic external genitalia as reported in female siblings with similar clinical presentation as Turner syndrome patients but had the X:autosome chromosome translocations (5).
Evaluation of amenorrhea
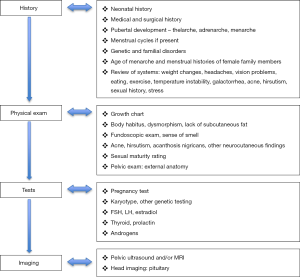
Figure 1 summarizes the recommended approach in evaluating amenorrhea. A vast history should be obtained, which may include questions regarding the following: stages of puberty, if any, that the patient has undergone (perhaps distinguishing a hypothalamic etiology), whether the patient has a family history of similar amenorrheic symptoms (suggesting a genetic cause), concerns regarding virilization (perhaps pointing toward adrenal causes or a 5-alpha reductase deficiency), and indications of increased stress or changes in diet and exercise (which may lead to functional hypothalamic amenorrhea. Information about chronic illnesses, dietary intake, level of physical activity, lifestyle changes, stress, and sexual activity obtained confidentially must be asked. Any new-onset symptoms of visual field changes and galactorrhea must be noted.
The physical examination requires review of growth parameters and trends overtime. Females with Turner syndrome are almost always short in stature. Assessing the sexual maturity rating in relation to growth and development provides further information on possible causes of amenorrhea. Particular skin findings, such as acne and hirsutism, can suggest hyperandrogenism. The genitourinary exam entails inspection of the genital area and an internal exam can be performed if indicated (6).
Pregnancy should initially be determined, most sensitively tested using serum beta human chorionic gonadotropin. A hormonal work-up is always helpful, and obtaining the FSH, TSH, prolactin, and serum androgen levels can identify specific etiologies. It is imperative to do imaging, usually a pelvic ultrasound, to determine presence or absence of anatomical structures in all females seen for primary amenorrhea. Subsequent evaluation may include karyotyping and pituitary imaging. A progesterone withdrawal challenge can be done, and a lack of response confirms suspicion of hypogonadotropic hypogonadism causes, commonly seen in females with significant weight loss and chronic illnesses (2,6).
Amenorrhea in polycystic ovary syndrome
In females presenting with either primary or secondary amenorrhea, PCOS is the most common endocrine disorder considered and affects 3.6–15% of reproductive age women (7). Different existing diagnostic criteria make determining prevalence rates challenging, and the most recent international guidelines recommended utility of the 2003 Rotterdam criteria for diagnosis (8). If a female meets any of the 2 diagnostic criteria—clinical or biochemical evidence of hyperandrogenism, anovulation or oligomenorrhea and ultrasound findings of polycystic ovarian appearance—a diagnosis of PCOS can be given.
The typical presentation is presence of menstrual irregularities which can be amenorrhea or oligomenorrhea usually associated with worsening acne and hirsutism. In adolescents, PCOS need to differentiated well in the setting of anovulatory cycles typical in the early post-menarchal years and elevated androgen levels common in pubertal development. In female adolescents who have attained full height and pubertal potential, and yet to have menarche, PCOS should be considered. Amenorrhea, primary or secondary, in PCOS occurs due to the blunted effects of hyperandrogenism on GnRH secretion resulting in elevated LH secretion. There are other neurotransmitters involved in the pathogenesis of PCOS such as neurokinin B and dynorphins that also affect LH activity (7).
To document excess androgens biochemically, testing for total and free testosterone, DHEAS, or androstenedione is warranted. Because testosterone has high affinity to sex hormone binding globulin, the latter is found to be low in PCOS cases with elevated free testosterone levels. The anti-mullerian hormone (AMH) correlates with ovarian reserve and is found to be increased in females with PCOS compared to those without PCOS. However, the AMH assay can be challenging to measure. Checking insulin levels is not necessary despite its pivotal role in the pathogenesis of PCOS. Clinically, hyperinsulinism is manifested with the presence of acanthosis nigricans. Known comorbidities of PCOS include obesity, type 2 diabetes mellitus, obstructive sleep apnea, mood disorders, and nonalcoholic fatty liver disease (7).
Amenorrhea in eating disorders
Amenorrhea is associated with eating disorders in 68% of cases (9). Although amenorrhea was removed from the diagnostic criteria for anorexia nervosa (AN) and bulimia nervosa (BN) in the DSM-5, approximately one-third of adolescents with eating disorders suffered from menstrual disorders (9). Table 2 summarizes the updated diagnostic criteria for AN and BN (10). Eating disorders can be associated with low body weight, excessive exercise, stress, and caloric restrictions, resulting in a negative energy balance that can predispose a woman to amenorrhea. Menstrual disorders have been associated with early onset eating disorders in adolescents which suggest correlation, if not causation. While low body weight and excessive exercise have been shown to predict secondary amenorrhea, the explanation why remains elusive as amenorrhea can persist even when these elements are modified (11).
Table 2
| Anorexia nervosa | Bulimia nervosa |
|---|---|
| 1. Energy restriction resulting in significantly low body weight. In children and adolescents, this results in halted pubertal development. |
1. Recurrent binge eating episodes |
| 2. Fear of weight gain with relating interfering behaviors (restriction, purging) | 2. Recurrent compensatory behaviors related to binge eating episodes |
| 3. Disturbed body image and perception | 3. Episodes ≥1/week for 3 months |
| 4. Body image concerns | |
| 5. Should be exclusive of AN episodes |
Patients with cystic fibrosis who have difficulties with appropriate nutrition and low body weight do not have the same predisposition to menstrual cycle disorders as patients with eating disorders, and thus low weight may not be the direct cause for amenorrhea (12). Up to 25% of patients with AN experience amenorrhea before significant weight loss occurs and resumption of menses does not occur immediately with appropriate weight gain (12).
Weight loss may be a predictor for amenorrhea in patients with AN, however the changes in the hypothalamic-pituitary axis may be the underlying explanation. Caloric restriction can cause suppression in the HPA resulting in changes in GnRH release, which reverts LH pulsatile release to pre-pubertal forms, culminating in cessation of pituitary production of LH and FSH. The lack of pulsatile secretions of LH and FSH leads to low levels of estrogen, and therefore ovulation does not occur (9). While nutritional rehabilitation and weight restoration help solve amenorrhea, they do not ensure resumption of menses. Although women with BN have lower incidence of secondary amenorrhea compared to women with AN, likely due to lower BMI experienced by women with AN, amenorrhea in BN is also thought to be caused by disruption of the HPA due to weight regulating behaviors (13,14).
Approximately 50% of adolescents with BN have hypothalamic dysfunction resulting in oligomenorrhea or menstrual irregularities. Patients suffering from bulimia do not experience the same drastically low body weight; they tend to have normal body weights, and therefore the menstrual disorders they experience are not caused by low body weight but due to nutritional restriction (12). Similar to the mechanism of action proposed to cause amenorrhea in patients with AN, the hypothalamic-pituitary dysfunction explains menstrual disorders in patients with BN (9).
The binge eating that occurs in BN may also contribute to hormonal imbalances via changes in insulin levels. Binge eating episodes are typically high in carbohydrates which results in high blood sugar and ultimately increased insulin release. Increased insulin levels can result in increases in testosterone thus leading to menstrual dysfunction via disruption of follicular maturation and ovulation (14). Bulimia has also been associated with polycystic ovarian syndrome (PCOS) which also has menstrual dysfunction as a result of insulin resistance-mediated increases in testosterone (14). While low weight can cause reproductive dysfunction due to hormonal changes, so too can obesity, also through hormonal changes (14).
Amenorrhea in female athletes
As female participation in sports and athletic activities continues to rise, understanding the subsequent health benefits and detriments becomes of significant importance. Within the population of female adolescent athletes, this idea of understanding the role of intense and competitive training is quite imperative, given the backdrop of adolescent hormonal changes and pubertal development taking place (15). With the increase in female adolescent physical activity came the demonstration of an associated spectrum of menstrual irregularities, ranging from subtle delays to amenorrhea (15,16).
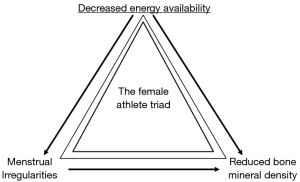
The female athlete triad (FAT) is a medical condition seen in girls and women involved in physical activities with three interconnected dysfunctions, though not mutually exclusive: menstrual irregularities as discussed above, reduced bone mineral density, and decreased energy availability (16). Figure 2 demonstrates the components of the triad in which a female athlete may present with 1 or more of the components in the triad. Menstrual dysfunction results from lack of energy availability, with both directly affecting bone health. While these dysfunctions range across various disciplines in sports, the risk of developing menstrual irregularities and the FAT does seem to be heightened in sports including figure skating, gymnastics, ballet, and long-distance running, where stress is placed on maintaining a specific lean weight (15). As a triad, and as separate entities, these health risks play important roles in the lives of female adolescents, and should be properly diagnosed and managed.
The way in which amenorrhea develops in the female adolescent athlete is no less than a disruption in a beautifully balanced system of hormonal regulation. Functional hypothalamic amenorrhea in the female adolescent athlete can arise due to a variety of factors, including decreased nutritional intake, decreased body weight and low relative body fat, intensity of sports training, and stress, among others (16). Recent studies have demonstrated that the greatest culprit in instigating the menstrual dysfunction is a negative energy balance between caloric intake and its expenditure during athletic training. Its ultimate effect is on diminishing the GnRH pulsatility, causing a decreased secretion of LH and FSH, which then disrupts ovarian hormonal production and leads to a decrease in estrogen and progesterone (15). Two other hormones that play a role in leading to the loss of GnRH pulsatility necessary for the maintenance of menstruation include leptin and ghrelin (15,17), together contributing to the idea of a disruption in energy balance.
Leptin, a hormone released by adipose tissue, is critical in maintaining pubertal development, acting as an influencer of overall energy maintenance and a maintenance factor of the GnRH pulsatile release (15,17). The hormone demonstrates a rise during pubertal stages, and has been shown to be decreased in the setting of low body fat and hypothalamic amenorrhea, thus contributing to the dysfunction seen (17). Ghrelin, an orexigenic peptide hormone significant in its role regarding energy balance, has been noted to be elevated in the setting of the functional amenorrhea seen in athletes, perhaps acting as a marker of energy insufficiency and increasing in a compensatory manner in these women. A cross-sectional study of adolescent amenorrheic and eumenorrheic athletes along with a control group of non-athletes demonstrated an overall decrease in fat mass, lower LH, and higher ghrelin secretion in the amenorrheic athletes, as well as an inverse association between percent body fat and levels of the ghrelin hormone (18).
The female adolescent athlete may experience a significant detriment in the context of bone mineral density, with major effects due to the decrease in estrogen production seen in functional hypothalamic amenorrhea as a result of the GnRH pulse-maker cessation. Adolescence is a crucial time in terms of bone growth and development of bone mass; however, with disruptions in the hypothalamic system comes disruptions in bone mineral density, leading to increased incidences of stress fractures and scoliosis in adolescent athletes with amenorrhea or delayed puberty (17). Interestingly, connections have also been made between this decreased bone density and other nutritional factors such as leptin, seen to be disrupted in amenorrheic athletes, leading to a possibility that the decrease in bone density seen may be a result of preservation in a negative energy balance state (17).
In discussing the long-term effects of amenorrhea in the female adolescent athlete, it is difficult to distinguish the consequences from those of a negative energy balance and nutritional deficit as well. Adolescents suffering from amenorrhea and the FAT have been noted to endure long-standing subsequent cardiac dysfunction including an increased risk for atherosclerosis with higher amounts of cholesterol and LDL. In addition, these adolescents are been noted to have an increase in musculoskeletal dysfunction with a lack of complete regeneration of the bone mineral density lost. In addition, psychological ramifications exist such as depression, anxiety, and detrimental changes in self-esteem (17).
Management of amenorrhea
The approach to managing females with amenorrhea is dictated by the underlying cause. In most cases, there is plausible treatment for amenorrhea. There is also the goal of achieving the normal physiologic levels for menstruation to occur and hopefully, deter future problems with fertility and bone health.
In polycystic ovary syndrome
Once the diagnosis of PCOS is established, the mainstay of treatment is oral contraceptives. These are effective in regulating menstrual cycles and lowering androgen levels. Additionally, since many PCOS females tend to be overweight or obese, weight reduction by healthy diet and exercise with or without pharmacologic intervention, such as metformin, are also recommended (7).
In eating disorders
Weight recovery is a therapeutic goal in ED, but there is no consensus concerning target weight (9). According to the American Psychiatric Association, the goals of nutritional rehabilitation for seriously underweight patients are to restore weight, normalize eating patterns, achieve normal perceptions of hunger and satiety, and correct biological and psychological sequelae of malnutrition. Unlike adults, for whom values such as ideal body weight or premorbid weight are used (19), there is no consensus about the precise target weight to be reached in adolescents with ED (11,12). At this young age, genetic and environmental factors, pubertal development, and continuous growth in various anthropometric parameters make clinical goals distinct in this population. There is a lack of consensus as to how to determine the treatment goal weight in the growing adolescent, when both height and weight are changing as part of normal development.
Many young females with ED may present to the obstetrician first due to menstrual abnormality. The American College of Obstetrics and Gynecologists (ACOG) recognizes the complexity of EDs and put forth recommendations to guide the specialists when caring for such a patient (20). It is not recommended to just treat the menstrual problem, but address the patient as a whole in collaboration with other members of the multidisciplinary team. As mentioned, weight restoration is the mainstay of treatment that eventually leads to restoration of periods and improvement of BMD. Calcium and vitamin D supplementation should be recommended. There is not enough evidence to support role of hormonal treatment in amenorrhea in ED patients especially in optimizing bone health.
In female athletes
Similar to the management of functional hypothalamic amenorrhea in females with ED, approach to the female adolescent athlete entails a multidisciplinary team, with emphasis on prevention of future lapses as well as treatment of current dysfunction (21). Namely, management employs an increase in fat and protein nutrition and a relative decrease in exercise to restore energy balance and maintain good energy availability for the adolescent. Utilization of a nutritionist can be helpful in determining the optimal caloric intake for the female adolescent athlete, while use of psychotherapy may be useful in treating an athlete with a previously unhealthy and difficult relationship with nutrition and weight, just as it may be useful in the treatment of certain eating disorders. Incorporation of increased calcium and vitamin D into the diet, through nutritional intake as well as pre-determined supplementation, may be warranted as well to optimize BMD. An intake of 1,500 mg/day of calcium and 1,500–2,000 IU/day of vitamin D is plausible (16).
Several methods are utilized to treat the adolescent athlete, with the caveat that bone mineral density must still be monitored and adequately managed. Oral contraception including the use of estrogen and cyclic progesterone (avoiding progesterone-only medications for possibility of increased bone loss) may return adolescents to a normal state of menstruation, but should absolutely continue to be monitored in terms of loss in bone density prior to medication administration (16,17,21). It is established as of now that bisphosphonates should not be utilized in the female adolescent as a means of managing osteopenia and osteoporosis symptoms, as their therapeutic effects have not yet been elucidated upon in this population (16,17).
Conclusions
Further investigation is warranted when a young adolescent presents with amenorrhea. It is crucial during evaluation to determine likely causes as existing treatment modalities may be available. Understanding the menstrual dysfunction due to common conditions, such as PCOS, ED and FAT, is necessary in providing care that is safe and evidence-based, hence preventing future negative health implications.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine for the Series “Adolescent Gynecology”. The articlehas undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.06.06). The series “Adolescent Gynecology” was commissioned by the editorial office without any funding or sponsorship. MDC served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Practice Committee of American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril 2008;90:S219-25. [Crossref] [PubMed]
- Klein DA, Poth MA. Amenorrhea: an approach to diagnosis and management. Am Fam Physician 2013;87:781-8. [PubMed]
- Greydanus D, Omar H, Tsitsika A, et al. Menstrual disorders in adolescent females. In: Omar H, Greydanus D, Tsitsika A, et al. (eds). Pediatric and adolescent sexuality and gynecology. New York: Nova Science Publishers Inc, 2010:315-411.
- Jean Emans S, DiVasta AD. Amenorrhea in the adolescent. In: Jean Emans S, Laufer MR (eds). Emans, Laufer, Goldstein’s Pediatric and Adolescent Gynecology. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2012:138-58.
- Omar HA, Hummel M, Jones EA, et al. Hypoplastic external genitalia in association with X;autosome chromosome translocation. J Pediatr Adolesc Gynecol 1999;12:161-4. [Crossref] [PubMed]
- Gray SH. Menstrual disorders. Pediatr Rev 2013;34:6. [Crossref] [PubMed]
- Dabadghao P. Polycystic ovary syndrome in adolescents. Best Pract Res Clin Endocrinol Metab 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome†‡. Hum Reprod 2018;33:1602-18. [Crossref] [PubMed]
- Vale B, Brito S, Paulos L, et al. Menstruation disorders in adolescents with eating disorders – target body mass index percentiles for their resolution. Einstein (São Paulo) 2014;12:175-80. [Crossref] [PubMed]
- Feeding and eating disorders. Diagnostic and Statistical Manual of Mental Disorders 2013. Available online: https://doi.org/
10.1176/appi.books.9780890425596.dsm10 , accessed 4 June 2019. - Abraham SF, Pettigrew B, Boyd C, et al. Predictors of functional and exercise amenorrhoea among eating and exercise disordered patients. Hum Reprod 2006;21:257-61. [Crossref] [PubMed]
- Weltman EA, Stern RC, Doershuk CF, et al. Weight and menstrual function in patients with eating disorders and cystic fibrosis. Pediatrics 1990;85:282-7. [PubMed]
- Martini MG, Solmi F, Krug I, et al. Associations between eating disorder diagnoses, behaviors, and menstrual dysfunction in a clinical sample. Arch Womens Ment Health 2016;19:553-7. [Crossref] [PubMed]
- Ålgars M, Huang L, Von Holle AF, et al. Binge eating and menstrual dysfunction. J Psychosom Res 2014;76:19-22. [Crossref] [PubMed]
- Márquez S, Molinero O. Energy availability, menstrual dysfunction and bone health in sports; an overview of the female athlete triad. Nutr Hosp 2013;28:1010-7. [PubMed]
- Berz K, McCambridge T. Amenorrhea in the female athlete: What to do and when to worry. Pediatr Ann 2016;45:e97-102. [Crossref] [PubMed]
- Stafford DEJ. Altered hypothalamic-pituitary-ovarian axis function in young female athletes. Treat Endocrinol 2005;4:147-54. [Crossref] [PubMed]
- Ackerman KE, Slusarz K, Guereca G, et al. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. Am J Physiol Endocrinol Metab 2012;302:E800-6. [Crossref] [PubMed]
- Heebink DM, Sunday SR, Halmi KA. Anorexia nervosa and bulimia nervosa in adolescence: Effects of age and menstrual status on psychological variables. J Am Acad Child Adolesc Psychiatry 1995;34:378-82. [Crossref] [PubMed]
- Committee on Adolescent Health Care. ACOG Committee Opinion No. 740: Gynecologic care for adolescents and young women with eating disorders. Obstet Gynecol 2018;131:e205-13. [Crossref] [PubMed]
- Committee on Adolescent Health Care. Committee Opinion No.702: Female athlete triad. Obstet Gynecol 2017;129:e160-7. [Crossref] [PubMed]
Cite this article as: Newbery G, Neelakantan M, Cabral MD, Omar H. Amenorrhea in adolescents: a narrative review. Pediatr Med 2019;2:30.