
Pediatric thoracic anesthesia: airway management for lung isolation and postoperative analgesia
Introduction
Thoracic surgery and relevant anesthetic techniques in children continue to advance. Two areas of particular importance that distinguish pediatric thoracic anesthesia from other pediatric anesthetic disciplines include approaches to one lung ventilation and specific regional anesthetic techniques to optimally manage postoperative pain in these patients. One lung ventilation techniques have evolved significantly over the past 25 years, particularly with the widespread availability and routine use of a number of different bronchial blockers which have in some cases replaced or provided an alternative to older approaches such as endobronchial intubation and double lumen endotracheal tubes (1-3). Additionally, there has been increased understanding and appreciation of the potential contributions of regional anesthetic techniques beyond local infiltration and neuraxial approaches to improving postoperative pain control. This brief review focuses on current airway management approaches to one lung ventilation in children and a number of various regional and multimodal strategies to optimally manage postoperative pain in children undergoing non-cardiac thoracic surgery.
One lung ventilation in children
Non-cardiac thoracic surgery in pediatric patients requires age or device specific strategies to achieve one lung ventilation. Due to the infrequency of these cases even in large children’s hospitals there are few if any prospective trials to guide the clinician in choosing an appropriate technique for one lung ventilation. As a result, much of the available literature on this topic is based on case reports or smaller case series (2-6).
Indications for one lung ventilation or lung isolation typically fall into two classes: absolute and relative. Absolute indications include, using lung isolation to prevent the soiling of one lung with blood or other contaminants originating from the other lung as a result of injury or infection on the effected side. Bronchopleural fistulas also represent an absolute indication as air may preferentially travel out a bronchopleural fistula on one side in the setting of positive pressure ventilation leading to inadequate inflation and thus inadequate ventilation on both sides requiring lung isolation to maintain adequate oxygenation and ventilation. Relative indications include the use lung isolation techniques to facilitate open or thoracoscopic procedures by deflating or isolating the lung on the operative side.
Physiology of one lung ventilation
The physiology of one lung ventilation can vary significantly when comparing adults with small children. In adults the combined mechanisms of hypoxic pulmonary vasoconstriction and the hydrostatic gradient created as a result of lateral positioning create a scenario of favorable ventilation/perfusion matching where blood is preferentially shunted to the dependent/ventilated lung (7). In children, because they are significantly smaller, the hydrostatic gradient is significantly less between the non-dependent and dependent lung in the lateral position leading to a more equal distribution of pulmonary blood flow even in the face of ongoing hypoxic pulmonary vasoconstriction. Additionally, distensibility of the chest wall, allows for compression the dependent lung and can further compromise ventilation leading to overall ventilation, perfusion mismatches (1,8). Ultimately, this may place the child at increased risk of intraprocedural hypoxemia. That said, a majority of children, even fairly small children appear to tolerate one lung ventilation in most clinical settings. The, exception to this tends to be in situations where there is an active or ongoing pneumonic process such as a necrotizing pneumonia which for a variety of reasons may substantially affect regional pulmonary blood flow and ventilation and perfusion thus increasing the risk of hypoxemia substantially during one lung ventilation.
Strategies for one lung ventilation
Techniques and device choice for one lung ventilation in children can vary significantly based on the size of the child and the comfort level of the clinician with a given device or technique. The most common techniques include: endobronchial or mainstem intubation, bronchial blocker placement, or placement of a double lumen tube.
Endobronchial intubation
Mainstem intubation or endobronchial intubation remains a common approach to achieving one lung ventilation in small children. In terms of advantages, the overall technique in most cases is fairly easy to accomplish. The primary concerns with this approach though, are the anatomic constraints of an infant’s tracheobronchial tree. In many cases, the mainstem bronchi are actually significantly smaller than the trachea at the level of the cricoid, to an extent, that an endotracheal tube that is appropriate for normal airway management may actually be too large to pass into a given mainstem bronchus. This is especially true when trying to place an endotracheal tube into the left mainstem bronchus which appears to be even smaller than the right mainstem bronchus in most children independent of age. As a consequence, many times the clinician may have to select an endotracheal tube that is a half size smaller than what might be considered appropriate based on the child’s age. A summary of tube sizes for endobronchial intubation is presented in Table 1.
Table 1
| Age | Endotracheal tube size |
|---|---|
| 0–5 months | 3.0 uc |
| 6–12 months | 3.0 c, 3.5 uc |
| 1 year | 3.5 c, 4.0 uc |
| 2 years | 3.5 c, 4.0 c |
| 3 years | 4.0 c |
| 4 years | 4.0 c |
c, cuffed; uc, uncuffed.
Strategies for placement include passing the endotracheal tube over a flexible fiberoptic scope into the desired mainstem bronchus, blind advancement with auscultation, or placement using fluoroscopy to direct the endotracheal tube into the desired bronchus (9). Drawbacks to the endobronchial approach include: an inability to rapidly switch from one lung ventilation to two lung ventilation, occlusion of the endotracheal tube lumen with blood or secretions leading to hypoventilation and hypoxemia (Figure 1), injury to the bronchus from an oversized endotracheal tube, and finally, clipping off of the upper lobe due to its proximity to the carina as the endotracheal tube is advanced into the desired bronchus leading to hypoventilation and potentially hypoxemia (Figure 2). The clinician should be aware though that this does tend to be much more common in cases where the clinician is passing the endotracheal tube into the right mainstem bronchus as the takeoff of the right upper lobe is typically in closer proximity to the carina when compared to the takeoff of the left upper lobe.
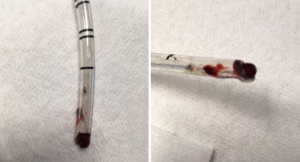
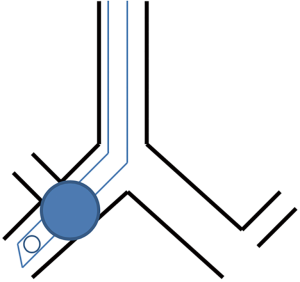
Bronchial blocker
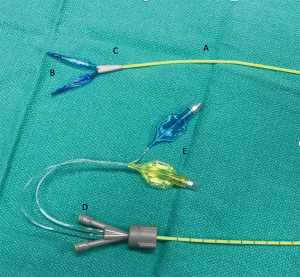
The other approach to lung isolation available in small children is the placement of a catheter based occlusive balloon which is inflated to occlude the mainstem bronchus on the operative side. At this time there are a number of bronchial blocker devices available including the Fogarty catheter (Edwards Life Sciences, Irvine, CA, USA), the Arndt (Cook Medical, Bloomington, IN, USA), the Fuji Uniblocker™ (Ambu, Columbia, MD, USA), the Univent™ Tube (Fuji Systems, Tokyo, Japan) tube, and the EZ-Blocker (Teleflex Inc., Wayne, PA, USA). Probably the most well documented of these is the Arndt bronchial blocker which is available in 3 sizes: 5 Fr, 7 Fr, and 9 Fr (Figure 3). Approaches to placement can vary by device but in young children placement is typically done extraluminally. Intraluminal placement is possible in larger children. A summary of device size, age, and the appropriateness of intraluminal/extraluminal placement is presented in Table 2.
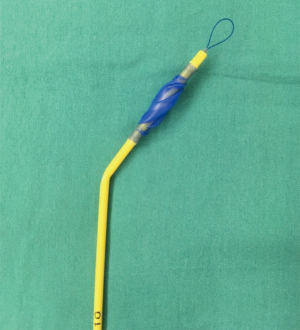
Table 2
| Age | Fogarty 3 Fr | Fogarty 4 Fr | Arndt 5 Fr | Arndt 7 Fr | Arndt 9 Fr | Fuji 5 Fr | Fuji 9 Fr | Cohen 9 Fr | EZ-Blocker 7 Fr |
|---|---|---|---|---|---|---|---|---|---|
| 0–5 months | E | E | E | E | |||||
| 6–12 months | E | E | E | E | |||||
| 1–2 years | E/I | E/I | E | E | |||||
| 3–7 years | E/I | E | E/I | E* | |||||
| 8–9 years | E/I | E | E | E | E | ||||
| 10–18 years | E/I | E/I | E/I | E/I |
*, published data in children over 6 years. Fogarty, Fogarty embolectomy catheter; Arndt, Arndt endobronchial blocker; Fuji, Fuji Uniblocker™; Cohen, Cohen endobronchial blocker; E, extraluminal; I, intraluminal.
Extraluminal placement of a bronchial blocker is accomplished by first inducing the patient and then performing a laryngoscopy. Typically, the blocker is placed first followed by an endotracheal tube into the patient’s glottis. Once in place, the blocker can be positioned either via direct vision with a flexible fiberoptic scope or via fluoroscopy. One of the primary technical considerations for accurate placement includes ensuring that there is adequate cephalad/caudad blocker mobility to allow for vertical translation of the blocker to allow for optimal positioning while at the same time allowing for adequate ventilation. At this time there are a number of excellent descriptions of varying approaches to placement and a detailed review of these is beyond the scope of this review (2,3,5).
Advantages of using a bronchial blocker include being able to rapidly transition from one lung ventilation to two lung ventilation and reliable isolation when correctly placed. Also, this technique can be executed in young children down to around 2.5 kg. Disadvantages include technical challenges with placement and intraprocedural migration of the device leading to loss of lung isolation and the need for intraprocedural replacement (3).
Other newer bronchial blocker-based devices available for use in children include the Univent™ tube and the EZ-Blocker (10-12). The Univent™ tube is a bronchial blocker that is basically attached to a single lumen endotracheal tube and the unit is placed via laryngoscopy while the blocker is advanced out of the device into the desired mainstem bronchus under direct vision with a flexible fiberoptic scope. Unfortunately, the large size of even the smallest Univent™ tube corresponds to the outer size of a 6.0 endotracheal tube limiting its use in children less than 6–7 years of age. The EZ-blocker, on the other hand, is a 7 Fr catheter-based device with two separate occlusive balloons designed to rest on the carina (Figure 4). Once in position one balloon or the other can be inflated to allow for isolation of the desired lung. Recent advances in extraluminal approaches have allowed this device to be used in children down to 6 years of age (10,11).
Double lumen tube
Double lumen endotracheal tubes remain a mainstay for executing one lung ventilation in adults. In children, however, their large size precludes their use in children less than 8 years of age, and older than this in some cases. To put the outer dimensions of the 26 Fr double lumen tube (the smallest double lumen tube available) in practical terms, the outer diameter is equivalent to the outer diameter of a 6.5 cuffed endotracheal tube which most clinicians would still consider to be oversized until around 10 or 11 years of age although some texts and reviews will suggest they can be used in younger children (13). Techniques for placement in size appropriate school aged children are similar to those described in adults. Advantages include reliable isolation, intraprocedural positional stability, an ability to rapidly transition for one to two lung ventilation, and ability to easily suction or apply continuous positive airway pressure in setting of refractory hypoxemia. Disadvantages include the potential for tracheal bronchial injury and the relatively large size of the device.
Postoperative pain management overview
Postoperative pain management is an essential part of the perioperative pathway for all patients undergoing thoracic surgery. Further, effective management strategies may, in addition to reducing pain and discomfort, curtail the rate of respiratory complications although there are no studies in pediatric patients to confirm this. One of the challenges to performing studies in pediatric patients to confirm the value of regional anesthesia in thoracic patients is that assessments of pain in young children are frequently difficult to quantitate in a meaningful and reproducible way requiring investigators to look at other surrogate outcomes like heart rate or return of bowel function which are less specific. In older children there are more options for self-reporting including the FLACC scale [i.e., the Face, Legs, Activity, Cry, ability to be Consoled (14)], behavioral assessments, and parental evaluation of their child’s discomfort. Given these challenges though, it is still important to regularly assess pain in these patients while in the hospital so that therapeutic adjustments can be made when necessary (15).
In the near postoperative period in a comprehensive multimodal approach to pain management, analgesics such as non-steroidal anti-inflammatory drugs (NSAIDS) and paracetamol should be administered on a regular schedule and not pro re nata (PRN) to ensure continuous therapeutic serum drug levels. This can be further supplemented by or improved upon with regional anesthetic techniques. In cases, however, where a regional anesthetic is not performed or fails to provide adequate analgesia, opioids should also be prescribed to help manage post procedure pain.
Loco-regional analgesia
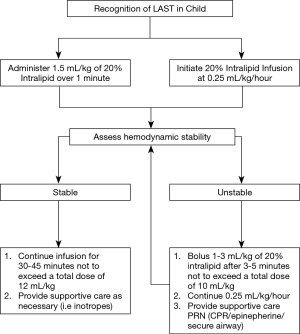
Today, loco-regional techniques in children are considered safe (16) and are frequently used for postoperative analgesia in a variety of procedural settings (17,18). Recent data from the Pediatric Regional Anesthesia Network (PRAN) has demonstrated that the risk of adverse events associated with regional blocks in children is comparable to that observed in adult patients (19). Unlike in adults though, frequently regional blocks in children are performed under general anesthesia to improve compliance and reduce patient movement during block placement. This practice is considered safe and has been endorsed as a routine practice standard by the European and American societies of regional anesthesia (ESRA and ASRA) (17). Interestingly, infants require higher relative doses of local anesthetic than adults to achieve nerve blockade. That said however, they are also at higher risk of neurologic or cardiac toxicity due to low plasma protein binding and decreased intrinsic clearance (16,20). Fortunately, the occurrence of local anesthetic systemic toxicity (LAST) in infants is rare but must be promptly treated with a 20% lipid emulsion (Figure 5). Given these conflicting issues, it is increasingly apparent that the clinician should not depend solely on a single analgesic strategy to optimally manage postoperative pain in pediatric thoracic patients but rather should implement a multimodal approach.
Epidural analgesia
Thoracic epidural analgesia (TEA) is commonly used in children undergoing non-cardiac thoracic surgery. The two most common approaches to catheter placement include direct placement via epidural needle insertion at T3 to T7 or via insertion of an epidural catheter at the level of the sacrococcygeal hiatus which is then threaded up under fluoroscopic guidance to the thoracic epidural space (Figure 6). Dosing approaches may include single bolus or continuous infusion of local anesthetics. In the direct approach to epidural catheter placement the epidural space is often reasonably straight forward to execute because of readily palpable landmarks, short distance between skin and epidural space, and favorable spinal column anatomy. Much like in adults, the clinician should use a loss of resistance to air or a loss of resistance to saline technique to identify the epidural space (17). In addition to these more traditional approaches, some centers are now employing ultrasound to facilitate epidural needle placement, using real time imaging to get a dynamic view of the needle and the catheter in vivo (21). Additionally, ultrasound has also been used to visualize caudal epidural catheter placement as well (22).
Commonly used local anesthetics for epidural infusion in pediatric patients include bupivacaine, ropivacaine, and/or levobupivacaine (Table 3). In addition to continuous infusions, ropivacaine can also be administered in a patient controlled epidural analgesia (PCEA) mode. An example of this would be to use a bolus dose 0.1 mL/kg with a lock-out interval of 10 minutes using one of the previously mentioned local anesthetics at a concentration of 0.1–0.2%, with a background infusion 0.1 mg/kg/h. Additionally, clonidine (0.5–1 mcg/mL), morphine (5–10 mcg/mL), or fentanyl (2–5 mcg/mL) can also be used as adjuvants in an epidural infusion solution.
Table 3
| Local anesthetic | Loading dose solution (%) | Loading dose (mL/kg) | Infusion solution (%) | Infusion limit (mg/kg/h) |
|---|---|---|---|---|
| Bupivacaine | 0.25 | 0.5 | 0.1–0.125 | <0.25–0.4 |
| Ropivacaine | 0.2 | 0.5 | 0.2 | 0.4 |
| Levobupivacaine | 0.25 | 0.5–1 | 0.0625–0.125 | 0.3 |
Thoracic paravertebral block (TPVB)
TPVBs are considered in many cases to be an alternative to TEA for patients undergoing thoracic surgery (23). The technique, like in adults, is typically performed under ultrasound guidance (24). Unfortunately, there is little data to guide the clinician in terms of the individual local anesthetic dose for each injection and further, there is little clarity in terms of the number of injection sites needed to achieve maximal analgesic effect. Recently, Vecchione et al. reported, as part of a larger series of 871 patients, their experience with 118 patients who received thoracic paravertebral catheters for postoperative pain management following thoracotomy (25). Additionally, they also reported their experience following single injection TPVBs in 35 patients who underwent video-assisted thoracoscopy. In their study they observed a major complication in only 1 patient who was not undergoing thoracic surgery. This complication was documented as a brief seizure and hypoxic episode following a bolus of local anesthetic. The overall rate of minor complications was 13.2%, although this was in all patients receiving a TPVB and not just those undergoing thoracic surgery. Minor complications included regional catheter dislodgement, occlusion or leakage of the regional catheter, minor bleeding at the insertion site, and in some cases skin irritation as a result of the catheter dressing. TPVB has also been used successfully for pain management in children after the Nuss procedure for pectus excavatum correction (26,27).
Intercostal nerve block (ICNB)
The ICNB is an older regional block that can be used for pain control after thoracotomy and video assisted thoracoscopic surgery. Intercostal nerve blocks have been previously performed to provide analgesia for chest tube insertion sites in both adults and children. In support of this, Lukosiene et al. showed that pediatric patients who received an ICNB had decreased rates of morphine consumption in the first 6 hours following chest tube placement (28). Additionally, intercostal nerve blocks have been shown to decrease opioid consumption in patients undergoing the Nuss procedure for pectus excavatum, and as a result, these patients also experienced a reduction in opioid related side effects (29).
Erector spinae plane block (ESPB)
The ESPB is a new technique first described in adults by Forero in 2016 (30). In this block, the local anesthetic is injected under ultrasound guidance into the interfascial plane deep to the erector spinae muscle at T5. This block is technically easy to execute in most cases and is presumed to be safer than TEA and TPVB because the injection site is far from the pleura and neuraxial structures. Further, there is a growing evidence for the efficacy of the ESPB for acute pain management following thoracic surgery in adults. In children however, there are still few reports to document placement and efficacy of this block although placement of a catheter and continuous ESPB block has been recently described in two case reports including a 7- and 15-month old infant undergoing thoracotomy (31,32).
Serratus anterior plane block (SAPB)
The SAPB is another newly described fascial block that aims to provide analgesia and/or anesthesia for thoracic wall surgery (33). The injection of the local anesthetic is performed between the posterior and midaxillary lines under ultrasound guidance and aims to block the intercostobrachial nerve, the lateral cutaneous branches of the intercostal nerves (T3–T9), the long thoracic nerve, and the thoracodorsal nerve located in a compartment between the serratus anterior and the latissimus dorsi muscles. The SAPB has been typically used for breast surgery but its popularity is growing for pain management following VATS procedures in adults and recently, the use of the SAPB has also been documented in a 10-year-old undergoing VATS (34). In further support of this, Kaushal et al. recently compared the efficacy of SAPB, Pectoral Nerves II (PECS II) block and ICNB for the management of postoperative thoracotomy pain after pediatric cardiac surgery and concluded that SAPB and PECS II are appropriate to use in children and further, likely decrease postoperative pain defined as a reduction in fentanyl consumption when compared to the other two regional techniques evaluated in the study (35).
Opioids
Opioids remain an important part of multimodal protocols for moderate to severe pain management after thoracic surgery. They are often combined with single shot locoregional technique including the ICNB or TPVB. Intravenous patient-controlled analgesia (PCA) is one of the most common and most effective strategies to administer postoperative opioids in these patients. In children, management of demand dosing with a PCA pump is typically reserved for patients older than 6 years of age because of issues of cognitive maturity. In younger children however, often a nurse or parent after a minimal degree of education can also function as a surrogate to assist younger patients in managing a PCA. Continuous basal opioid infusion is typically not advisable for initial pump programming but may be considered in cases of inadequate pain relief (36). In a recent meta-analysis of randomized trials in children the authors did not find any difference in opioid consumption, pain relief, or rate of adverse events between PCA with or without a background infusion, although the quality of evidence was poor due to a small overall sample size (37). Given this, the authors of this study recommend against the initial use of a background opioid infusion.
In terms of opiates appropriate for administration via a PCA morphine is the most commonly used drug for PCA administration worldwide, but pethidine, fentanyl, and hydromorphone are used as well. Morphine background infusion rates when used should typically be set to 0.01–0.03 mg/kg/h in patients who are relatively opioid naïve. In infants and children, the loading dose of morphine is typically between 0.05–0.1 mg/kg followed by PCA bolus dose of 0.01–0.03 mcg/kg with a lock-out interval between 6–10 minutes (38). Hydromorphone can also be administered on a scheduled or PRN basis at a dose of 5–15 µg/kg IV at 4–6 hours intervals. Similarly, oxycodone (0.1–0.2 mg/kg) or hydrocodone (0.05–0.1 mg/kg) can be administered orally at 4–6 hours intervals once the patient is able to take things by mouth. Tramadol represents another alternative to manage moderate pain and facilitate transitioning from other opiates on the second postoperative day. The usual dose is 1–2 mg/kg every 4–6 hours (intravenous or oral administration). Additionally, it can be used in place of one of the previously mentioned opiates in conjunction with a loco-regional technique.
NSAIDs and paracetamol
Regardless of whether or not the clinician has decided to employ a regional technique NSAIDs and paracetamol should be administered on a scheduled basis to form the base of a multimodal analgesic strategy as their analgesic effects have been demonstrated to decrease opioids consumption in many different perioperative settings. Further, there is no credible evidence to suggest that NSAIDs and specifically ketorolac increase the risk of bleeding after thoracic surgery. Ketorolac is typically administered in a loading dose of 0.5–1 mg/kg IV and is subsequently administered at a dose of 0.15–0.2 mg/kg every 6 hours up to 2 days (15). In the event that the intravenous route is unavailable ketorolac can be administered orally at a dose of 0.2 mg/kg every 4–6 hours as well. Ideally, paracetamol should be given by intravenous infusion over 15 minutes at a dose of 7.5 mg/kg in children weighing less than 10 kg and 15 mg/kg every 6 hours in children over 10 kg until postoperative day 3 (15).
NMDA antagonists, dexamethasone, and gabapentinoids
NMDA-receptor antagonists, like ketamine and magnesium sulphate can be used intraoperatively to reduce postoperative opioids consumption in the immediate postoperative period. Typical intravenous doses of ketamine tend to be between 0.25 and 0.5 mg/kg up to 20 mg. Dexamethasone can also be administered for both antiemetic and analgesic purposes at a dose of 0.15 mg/kg. In terms of gabapentinoids, such as gabapentin and pregabalin, there is little published data to guide the clinician on their use as part of multimodal analgesic strategy in children, although their use may represent a novel and effective alternative to further supplement multimodal approaches to analgesia in larger children.
Conclusions
All approaches to one lung ventilation have advantages and disadvantages and require some degree of practice and technical mastery to institute safely and effectively. In deciding on a technique or device the clinician should take care to weigh the risks and benefits of any one approach to one lung ventilation in a given patient. This also applies to the choice of loco-regional techniques and this is ultimately informed by the surgical approach and the clinician’s comfort level with a given regional technique. In truth, some new fascial block such as the ESB and SAPB may be safer than neuraxial approaches, but more experience is necessary to evaluate their true efficacy in these cases. Finally, a multimodal approach, comprised of NSAIDs, paracetamol and, if needed, opioids should be employed to ensure adequate pain relief in children following thoracic surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Scanagatta) for the series “Pediatric Thoracic Surgery” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.06.02). The series "Pediatric Thoracic Surgery" was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hammer GB. Single-lung ventilation in infants and children. Paediatr Anaesth 2004;14:98-102. [Crossref] [PubMed]
- Stephenson LL, Seefelder C. Routine extraluminal use of the 5F Arndt endobronchial blocker for one-lung ventilation in children up to 24 months of age. J Cardiothorac Vasc Anesth 2011;25:683-6. [Crossref] [PubMed]
- Templeton TW, Downard MG, Simpson CR, et al. Bending the rules: a novel approach to placement and retrospective experience with the 5 French Arndt endobronchial blocker in children <2 years. Paediatr Anaesth 2016;26:512-20. [Crossref] [PubMed]
- Marciniak B, Fayoux P, Hebrard A, et al. Fluoroscopic guidance of Arndt endobronchial blocker placement for single lung ventilation in small children. Acta Anaesthesiol Scand 2008;52:1003-5. [Crossref] [PubMed]
- Templeton TW, Lawrence AE, Lee AJ, et al. Inside out: reurposing endobronchial intubation to facilitate extraluminal placement of a 5 Fr Arndt bronchial blocker in young infants. Paediatr Anaesth 2018;28:668-9. [Crossref] [PubMed]
- Bird GT, Hall M, Nel L, et al. Effectiveness of Arndt endobronchial blockers in pediatric scoliosis surgery: a case series. Paediatr Anaesth 2007;17:289-94. [Crossref] [PubMed]
- Haynes SR, Bonner S. Anesthesia for thoracic surgery in children. Paediatr Anaesth 2000;10:237-51. [Crossref] [PubMed]
- Dunn PF. Physiology of the lateral decubitus position and one-lung ventilation. Int Anesthesiol Clin 2000;38:25-53. [Crossref] [PubMed]
- Kubota H, Kubota Y, Toyoda Y, et al. Selective blind endobronchial intubation in children and adults. Anesthesiology 1987;67:587-9. [Crossref] [PubMed]
- Piccioni F, Vecchi I, Spinelli E, et al. Extraluminal EZ-blocker placement for one-lung ventilation in pediatric thoracic surgery. J Cardiothorac Vasc Anesth 2015;29:e71-3. [Crossref] [PubMed]
- Templeton TW, Templeton LB, Lawrence AE, et al. An initial experience with an extraluminal EZ-Blocker: a new alternatice for one-lung ventilation in pediatric patients. Paediatr Anaesth 2018;28:347-51. [Crossref] [PubMed]
- Hammer GB, Brodsky JB, Redepath JH, et al. Use of the Univent tube for single-lung ventilation in paediatric patients. Paediatr Anaesth 1998;8:55-7. [Crossref] [PubMed]
- Seefelder C. Use of the 26-French double-lumen tube for lung isolation in children J Cardiothorac Vasc Anesth 2014;28:e19-21. [Crossref] [PubMed]
- Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 1997;23:293-7. [PubMed]
- Vittinghoff M, Lonnqvist PA, Mossetti V, et al. Postoperative pain management in children: Guidance from the pain committee of the European Society for Paediatric Anaesthesiology (ESPA Pain Management Ladder Initiative). Paediatr Anaesth 2018;28:493-506. [Crossref] [PubMed]
- Shah RD, Suresh S. Applications of regional anaesthesia in paediatrics. Br J Anaesth 2013;111:i114-24. [Crossref] [PubMed]
- Ivani G, Suresh S, Ecoffey C, et al. The European Society of Regional Anaesthesia and Pain Therapy and the American Society of Regional Anesthesia and Pain Medicine Joint Committee Practice Advisory on Controversial Topics in Pediatric Regional Anesthesia. Reg Anesth Pain Med 2015;40:526-32. [Crossref] [PubMed]
- Kendall MC, Castro Alves LJ, Suh EI, et al. Regional anesthesia to ameliorate postoperative analgesia outcomes in pediatric surgical patients: an updated systematic review of randomized controlled trials Local and Regional. Anesthesia 2018;11:91-109. [Crossref] [PubMed]
- Walker BJ, Long JB, Sathyamoorthy M, et al. Complications in Pediatric Regional Anesthesia. An Analysis of More than 100,000 Blocks from the Pediatric Regional Anesthesia Network. Anesthesiology 2018;129:721-32. [Crossref] [PubMed]
- Berde CB, Boretsky KR, Cravero JP. Paravertebral block for analgesia after pediatric thoracic surgery. Reg Anesth Pain Med 2014;39:179-80. [Crossref] [PubMed]
- Tsui BCH, Suresh S. Ultrasound Imaging for Regional Anesthesia in Infants, Children, and Adolescents A Review of Current Literature and Its Application in the Practice of Neuraxial Blocks. Anesthesiology 2010;112:719-28. [Crossref] [PubMed]
- Vecchione TM, Boretsky KR. Ultrasound Images of the Epidural Space through the Acoustic Window of the Infant. Anesthesiology 2017;126:126:562.
- Visoiu M. Paediatric regional anaesthesia: a current perspective. Curr Opin Anaesthesiol 2015;28:577-82. [Crossref] [PubMed]
- Boretsky K, Visoiu M, Bigeleisen P. Ultrasound-guided approach to the paravertebral space for catheter insertion in infants and children. Paediatr Anaesth 2013;23:1193-8. [PubMed]
- Vecchione T, Zurakowski D, Boretsky KR. Thoracic Paravertebral Nerve Blocks in Pediatric Patients: Safety and Clinical Experience. Anesth Analg 2016;123:1588-90. [Crossref] [PubMed]
- Hall Burton DM, Boretsky KR. A comparison of paravertebral nerve block catheters and thoracic epidural catheters for postoperative analgesia following the Nuss procedure for pectus excavatum repair. Paediatr Anaesth 2014;24:516-20. [Crossref] [PubMed]
- Qi J, Du B, Gurnaney H, et al. A prospective randomized observer-blinded study to assess postoperative analgesia provided by an ultrasound-guided bilateral thoracic paravertebral block for children undergoing the Nuss procedure. Reg Anesth Pain Med 2014;39:208-13. [Crossref] [PubMed]
- Lukosiene L, Macas A, Trepenaitis D, et al. Single shot intercostal block for pain management in pediatric patients undergoing the Nuss procedure: a double-blind, randomized, controlled study. J Pediatr Surg 2014;49:1753-7. [Crossref] [PubMed]
- Luo M, Liu X, Ning L, et al. Comparison of Ultrasonography-guided Bilateral Intercostal Nerve Blocks and Conventional Patient-controlled Intravenous Analgesia for Pain Control After the Nuss Procedure in Children A Prospective Randomized Study. Clin J Pain 2017;33:604-10. [Crossref] [PubMed]
- Forero M, Adhikary SD. The Erector Spinae Plane Block A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med 2016;41:621-7. [Crossref] [PubMed]
- Kaplan I, Jiao Y, AuBuchon JD, et al. Continuous Erector Spinae Plane Catheter for Analgesia After Infant Thoracotomy: A Case Report. A&A Practice. 2018;11:250-2. [Crossref] [PubMed]
- Gaio-Lima C, Costa CC, Moreira JB, et al. Continuous erector spinae plane block for analgesia in pediatric thoracic surgery: A case report. Rev Esp Anestesiol Reanim. 2018;65:287-90. [Crossref] [PubMed]
- Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia 2013;68:1107-13. [Crossref] [PubMed]
- Corso RM, Piraccini E, Byrne H, et al. The serratus anterior plane block for pediatric non-intubated video-assisted thoracoscopic surgery. Minerva Anestesiol 2017;83:775-6. [PubMed]
- Kaushal B, Chauhan S, Saini K, et al. Comparison of the Efficacy of Ultrasound-Guided Serratus Anterior Plane Block, Pectoral Nerves II Block, and Intercostal Nerve Block for the Management of Postoperative Thoracotomy Pain After Pediatric Cardiac Surgery. J Cardiothorac Vasc Anesth 2019;33:418-25. [Crossref] [PubMed]
- Grass JA. Patient-Controlled Analgesia. Anesth Analg 2005;101:S44-61. [Crossref] [PubMed]
- Hayes J, Dowling JJ, Peliowski A, et al. Patient-Controlled Analgesia Plus Background Opioid Infusion for Postoperative Pain in Children: A Systematic Review and Meta-Analysis of Randomized Trials. Anesth Analg 2016;123:991-1003. [Crossref] [PubMed]
- Yaster M, Andresini J, Krane EJ. Epidural analgesia. In: Yaster M, Krane EJ, Kaplan RF, et al. editors. Pediatric Pain Management and Sedation Handbook. St. Louis, Mosby, 1997:113-47.
Cite this article as: Piccioni F, Templeton TW, Morris B, Valenza F. Pediatric thoracic anesthesia: airway management for lung isolation and postoperative analgesia. Pediatr Med 2019;2:23.

