Primary spontaneous pneumothorax in children and adolescents: a systematic review
Introduction
The collection of air in the pleural space resulting in a partial or total lung collapse was firstly defined as “pneumothorax” by Laennec in 1813, but the first modern characterization of a primary spontaneous pneumothorax (PSP), the idiopathic variety occurring in absence of a pre-existing lung pathology, is attributed to Kjaergaard in 1932 (1). Despite the fact that it is very common among young healthy people, guidelines of spontaneous pneumothorax focus more on the adult population (2,3). Clinical practice for PSP has not changed in these years and is still dependent on the physicians’ custom. Lack of evidence about PSP management in children and adolescent patients has led to the adoption of different decision-making processes among specialists concerned with this pathology. The aim of this study is to summarize the knowledge gained in the last 30 years in order to review the optimal treatment of PSP in young population.
Search strategy and study selection
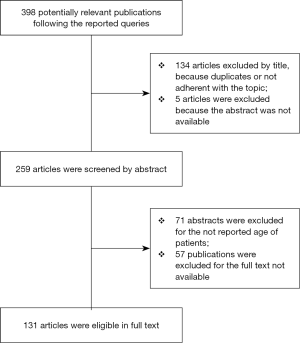
Our search strategy followed the PRISMA protocol guidelines (4). The authors independently reviewed the Embase and Medline databases for relevant articles about PSP on pediatric patients, published in English over the 30 years, from 1988 to 2018. An initial independent search with the following queries: ('primary spontaneous pneumothorax'/exp OR 'primary spontaneous pneumothorax' OR (primary AND spontaneous AND ('pneumothorax'/exp OR pneumothorax))) AND ([school]/lim OR [adolescent]/lim OR [young adult]/lim) AND [english]/lim AND [1988-2018]/py, ('paediatric primary spontaneous pneumothorax' OR (('paediatric'/exp OR paediatric) AND primary AND spontaneous AND ('pneumothorax'/exp OR pneumothorax)), ('pediatric primary spontaneous pneumothorax' OR (('pediatric'/exp OR pediatric) AND primary AND spontaneous AND ('pneumothorax'/exp OR pneumothorax)) was conducted and identified 398 potentially relevant publications were identified, 134 articles were excluded by title, because of duplicates or because they were not pertinent to the topic. The remaining abstracts were reviewed, and 71 publications did not report the age of patients. Of the remaining articles, 57 were excluded because the full text was not available and the abstract was listed as a congress presentation, and for 5of them the abstract was not available. In the end, 131 full-text articles were included in the present analysis. The selection process is shown in Figure 1. Additional articles and most recent guidelines were selected by authors from the references list of the reviewed publications.
Epidemiology
The exact incidence of PSP in pediatric population has not been reported but some authors agreed on an incidence rate of 3.4/100,000 (5) whereas in the general population the incidence range is between 6 and 18 per 100,000 each year and an increasing tendency has been recorded in the last few years (from 5.05/100,000 in 2001 to 7.18/100,000 in 2013) (6). The disease has been reported to have an initial peak among adolescents. In a large study on 19,562 patients aged 11 to 40 years, the aged-stratified incidence of PSP was analyzed (6). PSP showed a higher incidence after 14 years of age and decreased progressively after the age of 18 years, the peak of incidence was recorded at 15 years of age (>20/100,000), declining to the steady level at 25 years of age. Accordingly, with these data, an epidemiologic research on pneumothorax in England (primary and secondary combined) found an overall incidence of 11.1/100,000 per year for admissions to the emergency department (ED), with a double peak of incidence in two age groups, 15–34 and over 55 years, which overlaps the incidence of primary and secondary pneumothorax respectively (7). As expected, for gender distribution, this pathology is confirmed to have a male predominance as emphasized in other studies (5-9), but Poenaru et al. reported no difference in term of gender for children younger than 9 years of age (10,11). The recurrence rate for adolescents (up to 31%) is higher when compared to adults (<17% after 22 years of age). In child populations of <10 years old, the few available studies reported an incidence of PSP with rates between 0% and 15%, accordingly with the lower incidence of blebs and bullae (28%) at this age. It suggests that in childhood underlying congenital anomalies might be present as a predisposing factor (12,13). The common observation that PSP involves thinner and taller subjects with asthenic habitus and ectomorphic somatotype led several researchers to investigate body mass index (BMI) and the chest development in adolescents as a risk factor (14-16). BMI in PSP patient has been found to be lower when compared to the average value encountered in a control population (less of 20 kg/m2). A large study on teenagers with PSP from Taiwan indicated that the growth in term of vertical dimension of the thoracic cage in the pre-adolescents leads to a weakness on peripheral parenchyma of lungs. The result is the overdistension of the alveoli in the areas where the negative pressure is higher, as in the apex, and the formation of emphysematous-like lesions as blebs and bullae (6).
Physiopathology
The occurrence of PSP appears to be multifactorial and its exact etiology remains to be completely elucidated. The prevailing theory is based on the rupture of visceral subpleural blebs and bullae and of subsequent passage of air from the alveoli to intrapleural space (17,18). The pathogenesis of the bullae is related to a variety of factors including physical and environmental determinants. At the histopathological analysis on the surgical specimen, the pneumothorax-associated fibroblastic lesions (PAFL) are defined as a fibrosis located on the peripheral pleura classically associated with wedge-shaped septa and with fibroblastic foci disseminated on the visceral face. PAFL are not ubiquitous but were found in 50% of patients under 20 years of age and were absent in secondary pneumothoraces. Belchis et al. suggested that PAFL could be a component of a distinctive subtype of PSP (19). It is uncertain if PAFL are the result of a previous healing process or the expression of emphysematous-like changes (ELC) based on connective tissue disorders (20). The disruption of elastic fibers is the key to anomalies observed in the connective tissue and a relationship between elastolysis and an imbalance in the oxidative pathways has been investigated (21-23). Tobacco and cannabis smoke, as well as all chemical agents that have a direct inflammatory action on the distal airways, have been recognized as predisposing factors for lung injury and epithelial damage, playing an important role on the formation of ELC such as the bullous lesions (10,24,25). Park et al. analyzed the impact of the air pollutants on the admissions to the ED for PSP, finding that the increased concentration of O3, NO2, PM10, and PM2.5 implies an incidence rate of PSP increased approximately 15-, 16-, 3-, and 5-fold, respectively (26). The seasonal pattern of the onset of PSP, higher in spring and summer seasons, has been explained with atmospheric changes in term of pressure and temperature, but the exact impact of weather determinants is not clear (27-30).
Diagnosis
An episode of PSP must be suspected when, at rest or during a mild physical activity, an acute onset of pain occurs like a stab in the chest. Chest pain is well known as the most common presenting symptom (87% of patients) with dyspnea, present in 43%, and cough, seen in 5% of patients (31). Stronger symptoms in patients are not related to the degree of lung collapse and physical examination can be equivocal for smaller pneumothoraces. In this case the common described findings such an unequal breath sounds, hyperresonance with percussion over the chest wall and the anomaly of the wall movement on the affected side, can be unclear. The diagnosis of PSP must be confirmed by an upright chest X-ray (CXR) where the margins of the collapsed lung are evident. In the ED, current data in the literature indicate an increasing application of the ultrasonography (US) with PSP in the pediatric population. The results of a recent meta-analysis revealed a higher sensitivity and similar specificity in the use of ultrasonography compared with CXR. Pooled sensitivity and specificity were 0.88 and 0.99, respectively, for US, and 0.52 and 1.00, respectively, for CXR. Furthermore, because of its portability and the absence of ionizing radiations, some authors point out its usefulness especially for children and adolescents. On the other hand, the use of US as a reliable tool for the diagnosis of PSP, is limited because its accuracy is strongly dependent of the operator’s skill (32-34). The role of computed tomography (CT) scan for the first episode of PSP is controversial and generally this method is not considered a first-line diagnostic tool. CT scans are not encouraged by pediatricians and pediatric surgeons due to the risk of radiation exposure. This exposure has been calculated to be approximately 68 times the effective dose of a traditional CXR (35). A chest CT scan is used to detect the apical blebs and the pulmonary dystrophic areas, which are currently accepted as the cause of air leakage in PSP and are thought to be a risk factors for recurrence (35-38). The reported specificity of a routine chest CT scan (5 mm of slice thickness) in detecting parenchymal blebs ranges from 36% to 63% but a thin slice high-resolution CT (HRCT with 1 mm of slice thickness) offers a significantly greater sensitivity ranging between 94% and 97% (39,40). Nowadays there is still lack of evidence that the presence of blebs and bullae in the apical region of the lungs should select patients with a higher risk of recurrences and as a result, current arguments to support the use of CT scan in the management of PSP pediatric patients remain poor (41).
Management
The first aim in treating a pneumothorax is to allow the collapsed lung to re-expand. The method for achieving this goal is to relieve the pressure in the intrapleural space, draining out the air in order to avoid a tension pneumothorax that could lead to hemodynamic instability. The second aim may be to prevent recurrences. The choice of optimal treatment depends on the severity of the lung collapse, on the persistence of air leaks and on the clinical history of the patients. To calculate the size of the pneumothorax many methods have been proposed, but the accuracy of these equations has been questioned. The most used are the Light index, the Collins and the Rhea methods, all based on the estimated volume on the upright CXR (10). Accordingly, with the American College of Chest Physicians (ACCP) guidelines a pneumothorax is defined “large” when the distance between cupola and lung apex is >3 cm or, following British Thoracic Society (BTS) recommendations if the distance from the margin of the lung to the chest wall is >2 cm (2,3,42). After having defined the volume of the pneumothorax, two options are widely accepted for treatment: in conservative management, different choices are provided, from the simple observation with oxygen administration, to the aspiration of the intrapleural air through needle, pig-tail catheter and other types of chest tubes. Patients are eligible for conservative treatment at the first episode of PSP or if they are clinically stable and asymptomatic, with a small, non-hypertensive pneumothorax (<20% at Light index). The second option is surgical treatment that can be carried out through a pleurectomy, mechanical or chemical pleurodesis. These procedures must be proposed to patients affected by recurrences or by persistent air leaks resulting in a failed lung re-expansion after non-invasive therapy (43). Optimal timing to propose surgical treatment is still controversial (5,44). Some authors suggest that more of 50% of patients should be considered undertreated by chest tube only, reporting that 54% of conservatively resolved cases need surgery for PSP recurrence within 4 years. The proposal to adopt surgery as a first-choice treatment has been supported by Chambers and colleagues, Herrmann and colleagues, Morimoto and colleagues, with the assumption that videothoracoscopy guarantees better postoperative pain control and shorter hospital stay, resulting in a cost saving of 42% in comparison with conservative procedures (35,45-50). On the other hand, much progress has been made in conservative management as well, and an effective outpatient management has been developed by many protocols (42,51,52). Small-bore chest drains are attached to one-way devices (Heimlich valves, Tru-close thoracic vent) and the correct placement of the drain is documented by CXR. When a complete re-expansion of the lung is achieved in absence of air leaks, the patient is discharged from hospital with the chest tube secured with suture and dressings to prevent the kinking of the plastic drain. Control CXR was planned daily as an ambulatory follow-up assessment. The mean duration of catheter was 3.4±2.5 days. Of 50 patients initially discharged from ED with small drain and Heimlich valves 35 were never admitted to hospital and were fully managed with outpatient protocol (52). The outcomes of first-line conservative treatments among selected publications are summarized in Table 1.
Table 1
| Author [year] | Type of study | Age patients [n] | First-line treatment | Recurrence rate % |
|---|---|---|---|---|
| Aljehani [2018] | Retrospective | 18–30 [151] | CTD | CTD: 100 |
| Williams [2018] | Multicenter |
10–19 [1,040] | Obs/CTD/VATS | Obs failure: up to 33; CTD failure: up to 62; VATS: 4.3 |
| Williams [2017] | Retrospective | 12–21 [46] | CTD | CTD: 37 |
| Park [2017] | Retrospective | 15–23 [160] | Obs vs. O2 | N/A |
| Soler [2017] | Retrospective | 5–20 [81] | CTD/VATS | CTD: 39/ VATS: 6 |
| Herrmann [2015] | Retrospective | 10–89 [185] | VATS | VATS: 2.2 |
| Soccorso [2015] | Retrospective | 5–16 [50] | O2/CTD/Asp | Total: 18 |
| Lopez [2014] | Retrospective | 8–20 [96] | O2/CTD | O2/CTD failure: 37 |
| Robinson [2014] | Multicenter |
1–19 [155] | O2/Asp/VATS | O2/Asp: 95 |
| Seguier-Lipszyc [2011] | Retrospective | 10–18 [46] | O2/CTD | O2/CTD: 50 |
| Zganjer [2010] | Retrospective | 11–18 [16] | CTD | N/A |
| Lee [2010] | Retrospective | 14–18 [77] | Obs/Asp/CTD/VATS | Asp: 22/CTD: 46 |
| Shih [2010] | Retrospective | 15–18 [78] | Asp/CTD/VATS | Total: 35.9 |
| O'Lone [2008] | Retrospective | 1–18 [31] | Obs/CTD | Obs/CTD failure: 31 |
| Hui [2006] | Retrospective | 10–18 [63] | Asp/CTD | Asp: 26.3/CTD: 52.7 |
| Qureshi [2005] | Retrospective | 5–15 [43] | CTD/VATS | CTD: 54/VATS: 28.5 |
| Camuset [2005] | Prospective | 16–54 [35] | Asp | Asp: 49 |
| Noppen [2002] | Prospective |
16–54 [60] | Asp vs. CTD | Asp: 26/CTD: 27 |
| Weissberg [2000] | Retrospective | 11–86 [1,199/218 PSP] | CTD | CTD failure: 14 (VATS 3rd recurrence) |
| Wilcox [1995] | Retrospective | 2–16 [17] | Obs/CTD | CTD failure: 35 |
| Poenaru [1994] | Retrospective | 2–22 [58] | O2/CTD | CTD: >50 |
PSP, primary spontaneous pneumothorax; Asp, aspiration by needle; CTD, chest tube drain; Obs, simple observation; O2, oxygen supplementation; VATS, video-assisted thoracoscopic surgery; N/A, not applicable.
Needle aspiration
In pediatric patients the first-line attempt to resolve a PSP must be less invasive, as recommended by the current available guidelines (ACCP, BTS). The administration of oxygen in continuous flow (2–4 L/min) through non-rebreathing face mask or nasal cannula is the treatment of choice. The purpose of this procedure is based on a transpleural gradient which reduces the partial pressure of nitrogen on the alveolar side, and leads to a diffusion of nitrogen into the alveoli and a progressive resorption of the pneumothorax. Needle aspiration is accepted if small pneumothorax has a volume ranging between 20% and 40% with Light Index. Needle thoracocentesis is performed following various protocols by a simple manual aspiration with a 16-gauge needle inserted at the level of the second intercostal space crossing the midclavicular line (53-56). The pig-tail catheter with a diameter of 8-Fr can be placed by a Seldinger manoeuvre to repeat the aspiration. Air is manually aspirated with a syringe up to feel resistance. Control CXR is performed to check for complete lung re-expansion. If aspiration reaches 4,000 mL without re-expansion, a drain has to be inserted. It has been reported that needle aspiration is successful in 59.3% of cases with an immediate re-expansion of the lung, but 11% of the patients treated with simple aspiration needed tube drainage after 1 week and recurrence rates reached 26% for needle aspiration within the first year (56).
Chest drain
Chest drain insertion is performed at the second intercostal space on midclavicular line or at 4th or 5th intercostal space on the midaxillary line. The size of the drain has to be ranged between 16 and 24 Fr for the large-bore tubes and from 8 to 12 Fr for small-bore or pig-tail catheters. The best size for the chest catheter has been a matter of debate (56-60). The first study in a children’s population which proved the efficacy of the small-bore in comparison with large-bore drains was published in 2002 by Dull et al. (59). The results were favourable for the pain control but length of hospital stay (LOS) was not significantly different among the two groups. Kuo et al. (57) analyzed a cohort of 41 adolescents <18 years old treated with the conservative procedure. They found a success rate of more of 50% that was not dependent on the caliber of the tube. LOS and recurrence rates overlapped between the patients treated with small and large-bore drains. The authors pointed out the potential advantages of the small-bore drains because insertion does not require large incision on the chest wall, resulting in less pain and more esthetic wounds. There is no evidence on the utility of suction applied to drains. Lung re-expansion is achieved in up to 70% of patients with chest tube drainage alone by day 3 without suction. Some authors suggest that, when air leaks are present, only a small number of patients may benefit from suction and accordingly, these cases require more invasive procedures (10,57,61).
Surgical management
Surgical treatment is required when complete re-expansion of the lung cannot be achieved when conservative procedures fail, due to the persistence of air leakage or if pneumothorax recurs. The best timing to perform surgery is still a debated topic among surgeons (44-49). The definition of persistent air leaks differs among authors from 3 to 10 days and the decision to proceed with surgery can vary. This implies a prolongation of the hospital stay for patients who are monitored in the hospital with or without a chest tube placement (10). Video-assisted thoracoscopic surgery (VATS) is the procedure of choice because since its debut, it has resulted in low morbidity, better pain control and reduced recurrence rate for a shorter duration of the hospitalization (62-69). The goal of surgical treatment consists in the resection of blebs, bullae and fibrotic areas present on the pulmonary tissue, considered by many authors the source of the air leaks responsible of the pneumothorax, and in the stimulation of the pleurodesis through the formation of adhesions between pleural surfaces in order to avoid the collection of air in the intrapleural space and subsequent lung collapse. Different approaches for VATS have been developed through the past decades: from the initial technique with multiple accesses for the insertion of 2 or 3 thoracoports, a new technique based on single incision thoracoscopic surgery (SITS) has recently gained ground especially for pediatric patients (70-76). SITS is performed by a single incision, ranging from 5 to 20 mm, in the 7th intercostal space crossing the midaxillary line. All of instruments are introduced in the pleural cavity through the same access and for surgeons a previous experience with VATS is recommended so as not to cause movement drawback in the pleural cavity. After the introduction of the 30° camera, bullectomy and blebectomy are performed generally through a wedge resection by surgical stapler on the apex of the upper lobe of the lung where emphysematous lesions are more frequently found (77-79). Furthermore, apical stapling is usually done at the most suspected area even if no blebs could be clearly identified. Some author suggests that a resection of the apical segment of the lower lobe should be systematically performed because bullae at that level can be a potential risk for PSP recurrence after VATS (80). As alternative option to endostapler resection, ablation of blebs and bullae, with endoloop (81) with laser device (MBB 100 watt, Germany) (82) with the LigaSure vessel sealing system (LVSS) (83) (Valleylab, Boulder, CO., USA) and with radio-frequency device have also been described (84). Lee et al. along with Haraguchi and colleagues proposed a coverage of the staple line with absorbable materials as an additional procedure (85,86). In fact, the formation of the bullae in children and adolescents is a dynamic process caused by a weakness of visceral pleural and a lower elastic capacity of connective tissue that can be further compromised by the tension created around the staples after parenchymal resection. On the basis of intraoperative findings during redo surgery, new bullae were observed arising from the staple line (18,87). The coverage of the staples has been proposed to prevent the formation of new bullae on the weak pulmonary tissue (85,86). After the bleb resection, pleurodesis must be performed to maintain the lung adherent to the chest wall so as to prevent collapse in case of recurrence of pneumothorax. The first technique for pleurodesis was a complete parietal pleurectomy, which is not recommended for young patients due to the increased risk of bleeding and of intercostal nerve damage. Moreover, the tough pleural symphysis after pleurectomy can be an adjunctive difficulty in case of further thoracic intervention. Currently, the most consolidated techniques are the mechanical and the chemical pleurodesis (70,88). Mechanical pleurodesis is performed by an abrasion on pleural surface, using sterile materials such as gauze, swabs or scrapers through VATS access (89-91). Electric rotating brushes have also been described (92). The treatment is effective when hemorrhagic spots and mild bleeding appear from the parietal pleura. Pleural lesions can be made by electrocautery or argon beam as well, to decrease the risk of excessive bleeding that may happen with the abrasive technique (93). Chemical pleurodesis is performed by various agents with irritant and sclerosant properties: the most common are talc, iodopovidone, silver nitrate and minocycline (94,95). Minocycline has been introduced in clinical practice for its cost and its solubility which permits administration through the small-bore tubes, allowing outpatient management (95). On the other hand, a more intense chest pain has been observed after minocycline pleurodesis (96). Lillegard, Cobanoglu and Pathak recommended the autologous blood patch as a safe procedure to obtain an effective chemical pleurodesis and to resolve persistent air leaks specifically in children and adolescents (97-99). When associated with blebectomy, mechanical and chemical pleurodesis seem overlap in term of postoperative complication rates, outcomes and LOS, in pediatric and in the overall population, as showed by recent reviews and a meta-analysis (70,88,96,100).
Outcomes
According to the available data on PSP recurrences, it has been reported that without a surgical treatment, the rate is ranged between 16% and 52% after the first episode, rising to 60% after a second PSP and 80% following a third. The biggest proportion of recurrences (>60%) were observed within 2 years after the initial attack, with a reported risk (from 6% to 18%) of a pneumothorax in the contralateral lung (36,38,100,101). In the study by Choi et al. risks factors of PSP recurrence were retrospectively investigated in a population of 114 young patients under 18 years of age. It was recorded that after conservative treatment the recurrence rate for children and adolescents seems to be higher when compared to adult patients (50% vs. 30%). They did not find differences among the different types of treatment, whether with oxygen supplementation or chest tube placement (101). Moreover, the principal finding identified as risk factor, was the presence of blebs and bullae which is in accordance with other studies. The incidence of blebs/bullae discovered at HRCT scans in paediatric population was reported to range from 30.8% to 100% and the recurrence rate (from 50% to 100%) after conservative treatment is considered related to these “air-containing lesions” (102,103). Recurrence rates reported in case of presence of blebs/bullae identified by standard CT scan was 48%, compared to 20% when absent; on the other hand, some authors demonstrated that the rate of recurrence even in patients with a normal CT is high and is comparable to the recurrence rate in patients with blebs on CT. They showed that patients undergoing VATS had recurrence rates of 13%, compared to an average of 42% treated with chest drain only. Moreover, when blebs are discovered bilaterally, the overall risk of undergoing a contralateral VATS surgery is 28% (12,45,70). When surgery is necessary, the treatment of choice is based on VATS bullectomy and pleurodesis for low morbidity in the postoperative course, and, in comparison with conservative procedures, for the low risk of recurrence and for an overlapping LOS. In terms of LOS in literature the hospitalization rate in the largest series ranged between 2 to 11 days after VATS with a mean duration of 4 days, similar to patients treated with chest tube only. Functional short-term results at 12 months and long-term results at 5 years from surgery, showed an FEV1 >80% and unchanged DLCO in patients who underwent VATS blebectomy and talc pleurodesis (94,104,105). In a study on 41 patients, Bialas et al. reported an incidence of the recurrence rate of PSP after VATS blebectomy and mechanical or chemical pleurodesis of 5% (70). A study with a longer follow-up of 96 months recorded a recurrence rate after VATS surgery that overlapped with the results of other studies (from 0% to 5%). It has been shown that the different techniques of pleurodesis did not influence the recurrence rate: in a series of 596 patients for a total of 644 VATS procedures performed, Shaikhrezai et al. reported a success rate without further episodes of pneumothorax of 96.4%, 98.9%, 97.5% for abrasion, poudrage and pleurectomy respectively after a follow-up of 10 years (49,106,107). The reasons for recurrence after VATS surgery are the failure to recognize the source of the air leakage, when blebs are not seen, and an incomplete pleurodesis, particularly in correspondence to the site of trocar insertion. It has been reported that apical excision decreases the recurrence rate from 27% to 3% and in order to prevent postoperative prolonged air leaks, some authors suggest performing a resection of the apex of the lung even in absence of evident blebs (17). Other authors agreed that unseen blebs can represent a risk factor for postoperative prolonged air leaks and subsequent recurrence of pneumothorax, and they recommend the resection of the upper segment of the lower lobe where blebs can be identified during the 12.5% of VATS (70,80). When repeated surgery is required, the adherences formed after the previous pleurodesis can make the redo VATS more difficult and conversion to an open thoracotomy has to be considered. Doddoli et al. in a small series of 39 patients found that redo VATS is feasible in nearly 70% of cases. The conversion rate to thoracotomy was 39% and was found to be dependent only on the adherences after the previous pleurodesis, while the delay between the two interventions did not influence the feasibility of repeated VATS (87,108,109).
Discussion
An unequivocal agreement on the optimal strategy for PSP in pediatric population has not been reached yet. Currently, the guidelines based on adult patients, have been questioned for the paucity of evidence and for the lack of consistency around several management issues (37). In fact, the advances in diagnostic and in surgical technique did not correspond to an evolution in the management of PSP. Starting from the traditional definition, PSP has been historically labelled as idiopathic, because it arises in absence of an underlying pathology, in apparently healthy lung (110). In the pathologic findings of the resected blebs, the mechanism of elastogenesis has been investigated and a pattern of anomalies on the extracellular proteins was identified. This indicates a direct correlation between disorders of connective tissue and the incidence of PSP in patients younger than 25 years (19-23). These anomalies can be inherited [Marfan’s syndrome (13,20,111,112), Birt-Hogg-Dubè syndrome (113)] or acquired [anorexia (114), lower BMI (115), smoking habits (7)] but in any case they have not been taken into account as predisposing factors for a correct risk stratification of PSP recurrence and the options for treatment have not substantially changed over the years. In fact, indications for surgery for all patients are still the following (10): (I) second ipsilateral pneumothorax; (II) first contralateral pneumothorax; (III) bilateral pneumothorax; (IV) persistent air leak; (V) spontaneous haemothorax; (VI) professions (or intended professions) at risks for barotrauma. But, according to these recent analyses, a subgroup of population with a higher risk of PSP recurrence can be selected to benefit from a tailored treatment with surgery as first-line option. Some authors suggest that it is time to rethink the management of PSP (110). After its advent in 1990, VATS has become the surgical approach of choice (62): better outcomes in term of postoperative complications, control of pain after surgery, LOS and recurrence rate have also been reported in paediatric patients. An analysis of costs and benefits comparing VATS and open surgery identified a statistically significant difference in favor of VATS (68,69). These data have provided a substance to proposing VATS at the initial attack of PSP, but due to the lack of evidence to support this hypothesis, wide agreement among surgeons has not been reached yet (35,45-50). Moreover, a further evolution is in action in these years and SITS has gained ground among many authors (70-76). This technique promises to improve postoperative pain control and global outcomes but there are not prospective studies available yet that compare VATS and SITS. When the recurrence risk is not related to a recognizable lung disease, international guidelines still recommend the conservative approach as a first-line treatment for children and adolescents, but sensible variability can be found in current protocols (2,3,10-13). Three non-operative options are suggested: (I) needle aspiration, (II) pig-tail small-bore catheter and (III) large-bore tube thoracostomy. Even though these procedures are performed routinely with adults, tolerance is an aspect that has to be considered in pediatric patients, because it can be widely variable and often, the placement of drains may require sedation or even a general anesthesia. The effectiveness of needle manual aspiration is controversial for many authors and the placement of pig-tail catheter is usually preferred (116). Generally, these conservative procedures need in-hospital monitoring and its duration is dependent on physicians’ habits. Recently, outpatient protocols with small-bore drains connected to Heimlich valves have been introduced as an effective option with few complications. A systematic review of 18 studies of ambulatory management with Heimlich valves, reported an overall success rate of 86% and uneventful management in 78% of cases (117). In the future in-hospital management will be targeted to select patients for whom early surgery with a mini-invasive approach can be considered, due to the higher risk of PSP recurrence. For patients without predisposing factors, outpatient management with small chest catheters connected to one-way valve should, when possible, be proposed, in children and adolescents as well (110,118). In conclusion, PSP for pediatric patients needs involvement of pulmonologists, pediatricians, pediatric and thoracic surgeons, and this could be one of the reasons why the different points of view have prevailed regarding a common strategy for the treatment of disease. Further research, based on multidisciplinary task force statements, is required to provide stronger evidences for definitive protocols of this, until today, under-investigated pathology.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Scanagatta) for the series “Pediatric Thoracic Surgery” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.04.01). The series “Pediatric Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Papagiannis A, Lazaridis G, Zarogoulidis K, et al. Pneumothorax: an up to date "introduction". Ann Transl Med 2015;3:53. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58:ii39-52. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med 2009;6:e1000100 [Crossref] [PubMed]
- Lopez ME, Fallon SC, Lee TC, et al. Management of the pediatric spontaneous pneumothorax: is primary surgery the treatment of choice? Am J Surg 2014;208:571-6. [Crossref] [PubMed]
- Huang YH, Chang PY, Wong KS, et al. An Age-Stratified Longitudinal Study of Primary Spontaneous Pneumothorax. J Adolesc Health 2017;61:527-32. [Crossref] [PubMed]
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [Crossref] [PubMed]
- Mitani A, Hakamata Y, Hosoi M, et al. The incidence and risk factors of asymptomatic primary spontaneous pneumothorax detected during health check-ups. BMC Pulm Med 2017;17:177. [Crossref] [PubMed]
- Fujino S, Inoue S, Tezuka N, et al. Physical development of surgically treated patients with primary spontaneous pneumothorax. Chest 1999;116:899-902. [Crossref] [PubMed]
- Robinson PD, Cooper P, Ranganathan SC. Evidence-based management of paediatric primary spontaneous pneumothorax. Paediatr Respir Rev 2009;10:110-7; quiz 117. [Crossref] [PubMed]
- Poenaru D, Yazbeck S, Murphy S. Primary spontaneous pneumothorax in children. J Pediatr Surg 1994;29:1183-5. [Crossref] [PubMed]
- Seguier-Lipszyc E, Elizur A, Klin B, et al. Management of primary spontaneous pneumothorax in children. Clin Pediatr (Phila) 2011;50:797-802. [Crossref] [PubMed]
- Kaslow J, Bickel S, Wiesenauer C, et al. Pediatric Spontaneous Pneumothorax: Our Experience and a Review of the Literature. Pediatr Allergy Immunol Pulmonol 2018;31:208-14. [Crossref]
- Chang PY, Wong KS, Lai JY, et al. Rapid increase in the height and width of the upper chest in adolescents with primary spontaneous pneumothorax. Pediatr Neonatol 2015;56:53-7. [Crossref] [PubMed]
- Ayed AK, Bazerbashi S, Ben-Nakhi M, et al. Risk factors of spontaneous pneumothorax in Kuwait. Med Princ Pract 2006;15:338-42. [Crossref] [PubMed]
- Akkas Y, Peri NG, Kocer B, et al. A novel structural risk index for primary spontaneous pneumothorax: Ankara Numune Risk Index. Asian J Surg 2017;40:249-53. [Crossref] [PubMed]
- Ayed AK, Chandrasekaran C, Sukumar M. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: clinicopathological correlation. Eur J Cardiothorac Surg 2006;29:221-5. [Crossref] [PubMed]
- Ota H, Kawai H, Kuriyama S. The Presence of a Reticulated Trabecula-Like Structure Increases the Risk for the Recurrence of Primary Spontaneous Pneumothorax after Thoracoscopic Bullectomy. Ann Thorac Cardiovasc Surg 2016;22:139-45. [Crossref] [PubMed]
- Belchis DA, Shekitka K, Gocke CD. A unique, histopathologic lesion in a subset of patients with spontaneous pneumothorax. Arch Pathol Lab Med 2012;136:1522-7. [Crossref] [PubMed]
- Sauter JL, Butnor KJ. Pathological findings in spontaneous pneumothorax spec-imens: does the incidence of unexpected clinically significant findings justify routine histological examination? Histopathology 2015;66:675-84. [Crossref] [PubMed]
- Chen YW, Chiu WC, Chou SH, et al. High Nrf2 expression in alveolar type I pneumocytes is associated with low recurrences in primary spontaneous pneu-mothorax. Kaohsiung J Med Sci 2017;33:496-502. [Crossref] [PubMed]
- Chiu CY, Chen TP, Chen JR, et al. Overexpression of matrix metalloproteinase-9 in adolescents with primary spontaneous pneumothorax for surgical intervention. J Thorac Cardiovasc Surg 2018;156:2328-2336.e2. [Crossref] [PubMed]
- Hirai Y, Muragaki Y, Itoh S, et al. Fibulin-5 protein is reduced in the lung of patients with spontaneous pneumothorax who are under 25 years old. Ann Thorac Cardiovasc Surg 2012;18:200-5. [Crossref] [PubMed]
- Bintcliffe O, Maskell N. Spontaneous pneumothorax. BMJ 2014;348:g2928. [Crossref] [PubMed]
- Fiorelli A, Accardo M, Vicidomini G, et al. Does cannabis smoking predispose to lung bulla formation? Asian Cardiovasc Thorac Ann 2014;22:65-71. [Crossref] [PubMed]
- Park JH, Lee SH, Yun SJ, et al. Air pollutants and atmospheric pressure increased risk of ED visit for spontaneous pneumothorax. Am J Emerg Med 2018;36:2249-53. [Crossref] [PubMed]
- Motono N, Maeda S, Honda R, et al. Atmospheric temperature and pressure in-fluence the onset of spontaneous pneumothorax. Clin Respir J 2018;12:557-62. [Crossref] [PubMed]
- Oruç M, Şahin A, Dursun R, et al. Do Meteorological Changes Have an Effect on The Occurence of Spontaneous Pneumothorax? Turk Thorac J 2016;17:89-92. [Crossref] [PubMed]
- Haga T, Kurihara M, Kataoka H, et al. Influence of weather conditions on the onset of primary spontaneous pneumothorax: positive association with decreased atmospheric pressure. Ann Thorac Cardiovasc Surg 2013;19:212-5. [Crossref] [PubMed]
- Yamac ME, Karapolat S, Turkyilmaz A, et al. Relationship of spontaneous pneumothorax cases seen in Eastern Black Sea region with meteorological changes. Int J Biometeorol 2017;61:1493-8. [Crossref] [PubMed]
- Robinson PD, Blackburn C, Babl FE, et al. Paediatric Emergency Departments International Collaborative (PREDICT) research network. Management of paediatric spontaneous pneumothorax: a multicentre retrospective case series. Arch Dis Child 2015;100:918-23. [Crossref] [PubMed]
- Ding W, Shen Y, Yang J, et al. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest 2011;140:859-66. [Crossref] [PubMed]
- Unlüer EE, Karagöz A. Bedside ultrasonographic diagnosis of pneumothorax. Interv Med Appl Sci 2014;6:133-6. [Crossref] [PubMed]
- Ng C, Tsung JW. Point-of-care ultrasound for assisting in needle aspiration of spontaneous pneumothorax in the pediatric ED: a case series. Am J Emerg Med 2014;32:488.e3-8. [Crossref] [PubMed]
- Soler LM, Raymond SL, Larson SD, et al. Initial primary spontaneous pneumothorax in children and adolescents: Operate or wait? J Pediatr Surg 2018;53:1960-3. [Crossref] [PubMed]
- Guimaraes CV, Donnelly LF, Warner BW. CT findings for blebs and bullae in children with spontaneous pneumothorax and comparison with findings in nor-mal age-matched controls. Pediatr Radiol 2007;37:879-84. [Crossref] [PubMed]
- Soccorso G, Anbarasan R, Singh M, et al. Management of large primary sponta-neous pneumothorax in children: radiological guidance, surgical intervention and proposed guideline. Pediatr Surg Int 2015;31:1139-44. [Crossref] [PubMed]
- Choudhary AK, Sellars ME, Wallis C, et al. Primary spontaneous pneumothorax in children: the role of CT in guiding management. Clin Radiol 2005;60:508-11. [Crossref] [PubMed]
- Lee KH, Kim KW, Kim EY, et al. Detection of blebs and bullae in patients with primary spontaneous pneumothorax by multi-detector CT reconstruction using different slice thicknesses. J Med Imaging Radiat Oncol 2014;58:663-7. [Crossref] [PubMed]
- Chiu CY, Chen TP, Wang CJ, et al. Factors associated with proceeding to surgical intervention and recurrence of primary spontaneous pneumothorax in adolescent patients. Eur J Pediatr 2014;173:1483-90. [Crossref] [PubMed]
- Laituri CA, Valusek PA, Rivard DC, et al. The utility of computed tomography in the management of patients with spontaneous pneumothorax. J Pediatr Surg 2011;46:1523-5. [Crossref] [PubMed]
- Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med 2014;64:222-8. [Crossref] [PubMed]
- Sayar A, Kök A, Citak N, et al. A. Size of pneumothorax can be a new indication for surgical treatment in primary spontaneous pneumothorax: a prospective study. Ann Thorac Cardiovasc Surg 2014;20:192-7. [Crossref] [PubMed]
- Qureshi FG, Sandulache VC, Richardson W, et al. Primary vs delayed surgery for spontaneous pneumothorax in children: which is better? J Pediatr Surg 2005;40:166-9. [Crossref] [PubMed]
- Chambers A, Scarci M. In patients with first-episode primary spontaneous pneumothorax is video-assisted thoracoscopic surgery superior to tube thoracostomy alone in terms of time to resolution of pneumothorax and incidence of recurrence? Interact Cardiovasc Thorac Surg 2009;9:1003-8. [Crossref] [PubMed]
- Herrmann D, Klapdor B, Ewig S, et al. Initial management of primary spontaneous pneumothorax with video-assisted thoracoscopic surgery: a 10-year experience. Eur J Cardiothorac Surg 2016;49:854-9. [Crossref] [PubMed]
- Morimoto T, Fukui T, Koyama H, et al. Optimal strategy for the first episode of primary spontaneous pneumothorax in young men. A decision analysis. J Gen Intern Med 2002;17:193-202. [Crossref] [PubMed]
- Williams K, Lautz TB, Leon AH, et al. Optimal timing of video-assisted thoracoscopic surgery for primary spontaneous pneumothorax in children. J Pediatr Surg 2018;53:1858-61. [Crossref] [PubMed]
- Margolis M, Gharagozloo F, Tempesta B, et al. Video-assisted thoracic surgical treatment of initial spontaneous pneumothorax in young patients. Ann Thorac Surg 2003;76:1661-3; discussion 1663-4.
- Ganesalingam R, O'Neil RA, Shadbolt B, et al. Radiological predictors of recurrent primary spontaneous pneumothorax following nonsurgical management. Heart Lung Circ 2010;19:606-10. [Crossref] [PubMed]
- Kim YP, Haam SJ, Lee S, et al. Effectiveness of Ambulatory Tru-Close Thoracic Vent for the Outpatient Management of Pneumothorax: A Prospective Pilot Study. Korean J Radiol 2017;18:519-25. [Crossref] [PubMed]
- Hassani B, Foote J, Borgundvaag B. Outpatient management of primary spontaneous pneumothorax in the emergency department of a community hospital using a small-bore catheter and a Heimlich valve. Acad Emerg Med 2009;16:513-8. [Crossref] [PubMed]
- O'Lone E, Elphick HE, Robinson PJ. Spontaneous pneumothorax in children: when is invasive treatment indicated? Pediatr Pulmonol 2008;43:41-6. [Crossref] [PubMed]
- Park CB, Moon MH, Jeon HW, et al. Does oxygen therapy increase the resolution rate of primary spontaneous pneumothorax? J Thorac Dis 2017;9:5239-43. [Crossref] [PubMed]
- Camuset J, Laganier J, Brugière O, et al. Needle aspiration as first-line management of primary spontaneous pneumothorax. Presse Med 2006;35:765-8. [Crossref] [PubMed]
- Noppen M, Alexander P, Driesen P, et al. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. Am J Respir Crit Care Med 2002;165:1240-4. [Crossref] [PubMed]
- Kuo HC, Lin YJ, Huang CF, et al. Small-bore pigtail catheters for the treatment of primary spontaneous pneumothorax in young adolescents. Emerg Med J 2013;30:e17 [Crossref] [PubMed]
- Kelly AM, Kerr D, Clooney M. Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest 2008;134:1033-6. [Crossref] [PubMed]
- Dull KE, Fleisher GR. Pigtail catheters versus large-bore chest tubes for pneumothoraces in children treated in the emergency department. Pediatr Emerg Care 2002;18:265-7. [Crossref] [PubMed]
- Riber SS, Riber LP, Olesen WH, et al. The influence of chest tube size and position in primary spontaneous pneumothorax. J Thorac Dis 2017;9:327-32. [Crossref] [PubMed]
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321-35. [Crossref] [PubMed]
- Levi JF, Kleinmann P, Riquet M, et al. Percutaneous parietal pleurectomy for recurrent spontaneous pneumothorax. Lancet 1990;336:1577-8. [Crossref] [PubMed]
- Freixinet J, Canalis E, Rivas JJ, et al. Surgical treatment of primary spontaneous pneumothorax with video-assisted thoracic surgery. Eur Respir J 1997;10:409-11. [Crossref] [PubMed]
- Naunheim KS, Mack MJ, Hazelrigg SR, et al. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg 1995;109:1198-203; discussion 1203-4. [Crossref] [PubMed]
- Ayed AK, Al-Din HJ. The results of thoracoscopic surgery for primary spontaneous pneumothorax. Chest 2000;118:235-8. [Crossref] [PubMed]
- Körner H, Andersen KS, Stangeland L, et al. Surgical treatment of spontaneous pneumothorax by wedge resection without pleurodesis or pleurectomy. Eur J Cardiothorac Surg 1996;10:656-9. [Crossref] [PubMed]
- Massard G, Thomas P, Wihlm JM. Minimally invasive management for first and recurrent pneumothorax. Ann Thorac Surg 1998;66:592-9. [Crossref] [PubMed]
- Ayed AK, Jamal Al-Din H. Video-Assisted Thoracoscopy versus Thoracotomy for Primary Spontaneous Pneumothorax: A Randomized Controlled Trial. Med Principles Pract 2000;9:113-8. [Crossref]
- Waller DA, Forty J, Morritt GN. Video-assisted thoracoscopic surgery versus thoracotomy for spontaneous pneumothorax. Ann Thorac Surg 1994;58:372-6; discussion 376-7. [Crossref] [PubMed]
- Bialas RC, Weiner TM, Phillips JD. Video-assisted thoracic surgery for primary spontaneous pneumothorax in children: is there an optimal technique? J Pediatr Surg 2008;43:2151-5. [Crossref] [PubMed]
- Cho KD, Park CB, Cho MS, et al. Modification of thoracoscopic surgery for spontaneous pneumothorax. Asian Cardiovasc Thorac Ann 2006;14:472-5. [Crossref] [PubMed]
- Yamazaki K, Haratake N, Shikada Y, et al. Initial Experience of Single-Incision Thoracoscopic Surgery for 100 Patients with Primary Spontaneous Pneumothorax. Ann Thorac Cardiovasc Surg 2015;21:513-6. [Crossref] [PubMed]
- Yang HC, Kim S, Yum S, et al. Learning curve of single-incision thoracoscopic surgery for primary spontaneous pneumothorax. Surg Endosc 2017;31:1680-7. [Crossref] [PubMed]
- Ocakcioglu I, Alpay L, Demir M, et al. Is single port enough in minimally surgery for pneumothorax? Surg Endosc 2016;30:59-64. [Crossref] [PubMed]
- Prasad R, Arthur LG, Timmapuri SJ, et al. Early experience with single-incision thoracoscopic surgery in the pediatric population. J Laparoendosc Adv Surg Tech A 2011;21:189-92. [Crossref] [PubMed]
- Song IH, Yum S, Choi W, et al. Clinical application of single incision thoracoscopic surgery: early experience of 264 cases. J Cardiothorac Surg 2014;9:44. [Crossref] [PubMed]
- Chung PH, Wong KK, Lan LC, et al. Thoracoscopic bullectomy for primary spontaneous pneumothorax in pediatric patients. Pediatr Surg Int 2009;25:763-6. [Crossref] [PubMed]
- Casadio C, Rena O, Giobbe R, et al. Stapler blebectomy and pleural abrasion by video-assisted thoracoscopy for spontaneous pneumothorax. J Cardiovasc Surg (Torino) 2002;43:259-62. [PubMed]
- Stringel G, Amin NS, Dozor AJ. Video-assisted thoracoscopy in the management of recurrent spontaneous pneumothorax in the pediatric population. JSLS 1999;3:113-6. [PubMed]
- Obuchi T, Yoshida Y, Wakahara JI, et al. Pneumothorax in teenagers: reducing recurrence through resection of superior segment of lower lobe. J Thorac Dis 2018;10:3507-11. [Crossref] [PubMed]
- Takahashi R. Evaluation of Spontaneous Pneumothorax Surgeries: A 16-Year Experience in Japan. Surg Res Pract 2016;2016:7025793 [Crossref] [PubMed]
- Torre M, Grassi M, Nerli FP, et al. Nd-YAG laser pleurodesis via thoracoscopy. Endoscopic therapy in spontaneous pneumothorax Nd-YAG laser pleurodesis. Chest 1994;106:338-41. [Crossref] [PubMed]
- Li Z, Chen L, Wang J, et al. A single institution experience using the LigaSure vessel sealing system in video-assisted thoracoscopic surgery for primary spon-taneous pneumothorax. J Biomed Res 2014;28:494-7. [PubMed]
- Linchevskyy O, Makarov A, Getman V. Lung sealing using the tissue-welding technology in spontaneous pneumothorax. Eur J Cardiothorac Surg 2010;37:1126-8. [Crossref] [PubMed]
- Lee S, Kim HR, Cho SKorean Pneumothorax Study Group, et al. Staple line coverage after bullectomy for primary spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:2005-11. [Crossref] [PubMed]
- Haraguchi S, Koizumi K, Mikami I, et al. Staple line coverage with a polyglycolic acid sheet plus pleural abrasion by thoracoscopic surgery for primary spontaneous pneumothorax in young patients. J Nippon Med Sch 2012;79:139-42. [Crossref] [PubMed]
- Cho S, Jheon S, Kim D, et al. Results of repeated video-assisted thoracic surgery for recurrent pneumothorax after primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2018;53:857-61. [Crossref] [PubMed]
- Vuong NL, Elshafay A, Thao LP, et al. Efficacy of treatments in primary spontaneous pneumothorax: A systematic review and network meta-analysis of randomized clinical trials. Respir Med 2018;137:152-66. [Crossref] [PubMed]
- Chan P, Clarke P, Daniel FJ, et al. Efficacy study of video-assisted thoracoscopic surgery pleurodesis for spontaneous pneumothorax. Ann Thorac Surg 2001;71:452-4. [Crossref] [PubMed]
- Rena O, Massera F, Papalia E, et al. Surgical pleurodesis for Vanderschueren's stage III primary spontaneous pneumothorax. Eur Respir J 2008;31:837-41. [Crossref] [PubMed]
- Gossot D, Galetta D, Stern JB, et al. Results of thoracoscopic pleural abrasion for primary spontaneous pneumothorax. Surg Endosc 2004;18:466-71. [Crossref] [PubMed]
- Maier A, Anegg U, Renner H, et al. Four-year experience with pleural abrasion using a rotating brush during video-assisted thoracoscopy. Surg Endosc 2000;14:75-8. [Crossref] [PubMed]
- Bobbio A, Ampollini L, Internullo E, et al. Thoracoscopic parietal pleural argon beam coagulation versus pleural abrasion in the treatment of primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2006;29:6-8. [Crossref] [PubMed]
- Dubois L, Malthaner RA. Video-assisted thoracoscopic bullectomy and talc poudrage for spontaneous pneumothoraces: effect on short-term lung function. J Thorac Cardiovasc Surg 2010;140:1272-5. [Crossref] [PubMed]
- Chen JS, Chan WK, Tsai KT, et al. Simple aspiration and drainage and intrapleural minocycline pleurodesis versus simple aspiration and drainage for the initial treatment of primary spontaneous pneumothorax: an open-label, parallel-group, prospective, randomised, controlled trial. Lancet 2013;381:1277-82. [Crossref] [PubMed]
- Ling ZG, Wu YB, Ming MY, et al. The effect of pleural abrasion on the treatment of primary spontaneous pneumothorax: a systematic review of randomized controlled trials. PLoS One 2015;10:e0127857 [Crossref] [PubMed]
- Lillegard JB, Kennedy RD, Ishitani MB, et al. Autologous blood patch for persistent air leak in children. J Pediatr Surg 2013;48:1862-6. [Crossref] [PubMed]
- Cobanoglu U, Melek M, Edirne Y. Autologous blood pleurodesis: A good choice in patients with persistent air leak. Ann Thorac Med 2009;4:182-6. [Crossref] [PubMed]
- Pathak V, Quinn C, Zhou C, et al. Use of autologous blood patch for prolonged air leak in spontaneous pneumothoraces in the adolescent population. Lung India 2018;35:328-31. [Crossref] [PubMed]
- Alayouty HD, Hasan TM, Alhadad ZA, et al. Mechanical versus chemical pleurodesis for management of primary spontaneous pneumothorax evaluated with thoracic echography. Interact Cardiovasc Thorac Surg 2011;13:475-9. [Crossref] [PubMed]
- Choi SY, Du Kim Y, Kim DY, et al. Influence of lung resection volume on risk of primary spontaneous pneumothorax recurrence. J Thorac Dis 2018;10:1622-7. [Crossref] [PubMed]
- Young Choi S, Beom Park C, Wha Song S, et al. What factors predict recurrence after an initial episode of primary spontaneous pneumothorax in children? Ann Thorac Cardiovasc Surg 2014;20:961-7. [Crossref] [PubMed]
- Uramoto H, Shimokawa H, Tanaka F. What factors predict recurrence of a spontaneous pneumothorax? J Cardiothorac Surg 2012;7:112. [Crossref] [PubMed]
- Williams K, Oyetunji TA, Hsuing G, et al. Spontaneous Pneumothorax in Children: National Management Strategies and Outcomes. J Laparoendosc Adv Surg Tech A 2018;28:218-22. [Crossref] [PubMed]
- Cardillo G, Carleo F, Carbone L, et al. Long-term lung function following video-thoracoscopic talc poudrage for primary spontaneous recurrent pneumothorax. Eur J Cardiothorac Surg 2007;31:802-5. [Crossref] [PubMed]
- Grewal H, Jackson RJ, Dunton RF, et al. Video-assisted thoracic surgery is safe and effective in the treatment of spontaneous pneumothorax in children. Pediatric Endosurgery and Innovative Techniques 2001;5:365-70. [Crossref]
- Shaikhrezai K, Thompson AI, Parkin C, et al. Video-assisted thoracoscopic surgery management of spontaneous pneumothorax--long-term results. Eur J Cardiothorac Surg 2011;40:120-3. [Crossref] [PubMed]
- Doddoli C, Barlési F, Fraticelli A, et al. Video-assisted thoracoscopic management of recurrent primary spontaneous pneumothorax after prior talc pleurodesis: a feasible, safe and efficient treatment option. Eur J Cardiothorac Surg 2004;26:889-92. [Crossref] [PubMed]
- Imperatori A, Rotolo N, Spagnoletti M, et al. Risk factors for postoperative recurrence of spontaneous pneumothorax treated by video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2015;20:647-51; discussion 651-2. [Crossref] [PubMed]
- Bintcliffe OJ, Hallifax RJ, Edey A, et al. Spontaneous pneumothorax: time to rethink management? Lancet Respir Med 2015;3:578-88. [Crossref] [PubMed]
- Karpman C, Aughenbaugh GL, Ryu JH. Pneumothorax and bullae in Marfan syndrome. Respiration 2011;82:219-24. [Crossref] [PubMed]
- Weissberg D, Refaely Y. Pneumothorax: experience with 1,199 patients. Chest 2000;117:1279-85. [Crossref] [PubMed]
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007;175:1044-53. [Crossref] [PubMed]
- Loung RP, Cooney M, Fallon EM, et al. Pneumothorax in a young man with anorexia nervosa. Int J Eat Disord 2016;49:895-8. [Crossref] [PubMed]
- Tan J, Yang Y, Zhong J, et al. Association Between BMI and Recurrence of Primary Spontaneous Pneumothorax. World J Surg 2017;41:1274-80. [Crossref] [PubMed]
- Baumann MH, Strange C. Treatment of spontaneous pneumothorax: a more aggressive approach? Chest 1997;112:789-804. [Crossref] [PubMed]
- Brims FJ, Maskell N. Ambulatory treatment in the management of pneumothorax: a systematic review of the literature. Thorax 2013;68:664-9. [Crossref] [PubMed]
- Kepka S, Dalphin JC, Pretalli JB, et al. How spontaneous pneumothorax is managed in emergency departments: a French multicentre descriptive study. BMC Emerg Med 2019;19:4. [Crossref] [PubMed]
Cite this article as: Furia S, Breda C. Primary spontaneous pneumothorax in children and adolescents: a systematic review. Pediatr Med 2019;2:12.