
Pulmonary metastasectomy in children and adolescents: a narrative review
Introduction
Nearly a quarter of children with solid tumors has a metastatic disease at their initial diagnosis, while another 20% have a metastatic onset during or after treatment; with the most common site of these metastases being in the lung (1). The role of surgery in metastatic disease depends greatly on the primary-tumor histology. As a general rule, tumors that are refractory to adjuvant therapies are most appropriate for a pulmonary metastasectomy.
Methods
An electronic search was performed for relevant studies from January 1950 to November 2018 in the following databases: PubMed, Science Direct, Embase, Scopus and Cochrane. A combination of search terms included “pulmonary metastasectomy, pediatric tumors, pediatric cancer”; the reference list also included articles identified manually and studies referenced for other purposes.
General indications
The role of metastasectomy has been actively investigated in adult patients, increasing the 5-year survival from 10% to 40–55% (2).
However, studies on children and adolescents are less numerous because of the rarity of pediatric solid tumors, and so patients with a metastatic pulmonary disease which could be considered for surgery are very few; in these selected cases indication for surgery should be evaluated by the multidisciplinary study team, in selected hospitals and institutions. Surgeons, pediatric oncologists, radiotherapists, psychologists, and radiologists contribute with their different skills to the management of pulmonary metastases. Indications to perform pulmonary metastasectomy differ according to primitive histology (Table 1).
Table 1
| Histology | Indications | Notes |
|---|---|---|
| Osteosarcoma (3-9) | PM mandatory to achieve LTS | Thoracotomy allows detection of CT-occult nodules in 25% to 35% of patients |
| Patients with lung-only metastases | Longer DFS and better histologic ChT response | |
| NRST (4,8,10-13) | PM should be performed if CR is possible | Generally ChT and RT resistant |
| Nodules are soft; hence localization techniques may be useful | Metastases are mostly pulmonary | |
| Rhabdomyosarcoma (4,8,14) | PM reserved for diagnosis (poor outcome and good response to ChT and RT) | High risk of relapse after PM |
| Adrenocortical carcinoma (8,15-17) | Case series describe LTS after PM in adults | ChT and RT resistant. |
| Extremely rare, case reports describe LTS after PM in the pediatric population | Risk of implants and carcinomatosis due to tumor rupture during surgery | |
| Neuroblastoma (8,18,19) | Pulmonary metastases are rare (3.6%) | Age older than 1 year and MYCN amplification (high-risk group) are more likely to have metastases |
| PM reserved for diagnosis (if the biopsy is not possible) | ||
| Ewing’s sarcoma (8,20-22) | Diagnostic role of PM with no proven effect on survival | ChT and RT-sensitive |
| Surgery may be useful in selected cases to avoid further ChT or RT | ||
| Hepatoblastoma (8,23-25) | PM indicated for residual disease after ChT | CR of both primitive and metastases is essential for LTS |
| PM should be performed if CR is possible | ||
| PM should be performed before liver transplantation | ||
| Wilms’ tumor (8,26-30) | PM for residual nodules after ChT | ChT and RT-sensitive |
| PM can avoid unnecessary RT/ChT | Whole lung RT can lead to interstitial lung disease and secondary malignant tumor (i.e., breast cancer) |
PM, pulmonary metastasectomy; LTS, long term survivor; DFS, disease-free survival; CT, computed tomography; NRST, non-rhabdomyosarcoma soft tissue sarcoma; ChT, chemotherapy; RT, radiotherapy; CR, complete resection.
Surgical removal of lung deposits is a time-consuming, demanding surgical treatment for pediatric metastatic tumors. Single cases and few case-series experiences are reported starting from the 1950s. Case-series became numerically significant in the 1970s, and most of them included different types of tumors (31-33), and the spreading and progressive qualitative improvement of chest computed tomography (CT) led to a more sensitive preoperative identification of pulmonary nodules (Figure 1A). In this scenario, the multidisciplinary teams, with pediatric-thoracic surgeons as the main character, have accepted the idea that children could tolerate a single, sequential staged or even repeated thoracotomy (after further relapses). Lung volume loss has been faced using non-anatomic parenchymal-sparing resections (wedge or precision resections). Ranges of overall survival vary from 20% to 70% for patients with metastatic disease, depending on primary histology (Table 1).
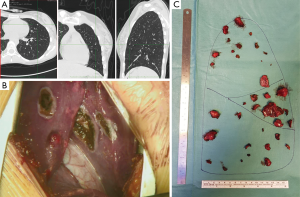
Technical aspects
After accurate cardio-pulmonary evaluation, patients undergo general anesthesia and are intubated with a double-lumen tube in order to obtain intra-operative single-lung ventilation.
For adolescents a lung collapse is achievable with smaller double-lumen tubes, which usually requires the aid of a positioned pediatric bronchoscope. For younger children, conventional double-lumen tubes are too large, and the positioning of a bronchial blocker is preferred. The blocker is positioned under the supervision of a pediatric bronchoscope, obtaining a selective bronchial exclusion.
The complete lung’s collapse allows for the whole lung palpation and the detection of chest-CT occult nodules. In literature, different accesses to the thoracic cavity have been proposed in order to obtain a complete resection from thoracotomy to sternotomy to video-assisted thoracoscopic surgery (VATS) (34).
In our experience, we usually prefer using a minimally-invasive complete muscle-sparing lateral thoracotomy, even in cases of a planned staged thoracotomy (35).
The patient is positioned on lateral decubitus, exposing the lateral chest wall by arm abduction on an arch or a pillow.
Nodules are removed through precision resections using electrocautery or laser methods in order to ensure radical surgery with adequate margins while preserving the surrounding parenchyma, thus causing a limited volumetric distortion as compared to staplers (Figure 1B,C). Parenchymal defects are closed by single or bidirectional locked suture of 3-0 of polypropylene. When there are several resections, it is impossible to close every defect without impairing postoperative lung function and expansion. Therefore, in these patients, we prefer to close only the deeper defects leaving the more superficial ones opened, covering them with autologous fat tissue grafting, applying commercial aerostatic fibrin-glues or using patches in order to reduce the air leaks (3,36,37).
The use of staplers to perform a wedge resection should be reserved for patients with few, localized lesions. In children, the kind of stapler depends on the thickness of the pulmonary parenchyma and the cartridges. They may vary from 2.5 mm for peripheral nodules to 3.5 mm for more central wedge resections or for closing a central bronchus.
Anatomical resections (segmentectomy, lobectomy or pneumonectomy) should be reserved for selected cases since a major lung resection can only be justified if it leads to a real oncological advantage.
In the presence of pleural deposits, pleurectomy is not indicated in most pediatric tumors. When there is the suspect of pleural disease, an intra-operative histologic evaluation through frozen-section should be performed to decide whether the procedure should be carried on.
Post-operative pain control can be obtained using an epidural catheter, which can be placed before anesthesia induction or, in young children, at the end of the operation. If the epidural catheter is refused or not feasible, intravenous drugs can be administered using an elastomeric pump or by patient-controlled analgesia (PCA) in motivated and collaborative older children and adolescents (35).
Metastasectomy should be planned with the intent to perform a complete resection of all the nodules. Partial debulking is admitted only in the case of an intra-operative evaluation of non-operability after having performed an initial resection.
Repeated thoracotomies following relapses should be evaluated. In these cases, the planning of surgical intervention should be carefully considered along with the previous procedures performed with the location and characteristics of present recurrence. There is no theoretical limit for re-interventions, as feasibility depends more on residual respiratory function and on the natural history of the disease, rather than on technical difficulties; however, these cases should be referred only to specialized oncologic centers.
Outcomes and conclusions
Peri-operative mortality is rarely reported while surgical complications rate can vary from 0–12% (4,5,38) including air-leaks, pneumonia, respiratory insufficiency, and superficial wound infection.
The only frequent issue is the management of chest tubes and respiratory physiotherapy in the presence of reduced residual lung expansion due to the iatrogenic inelasticity of resected and sutured areas. In these cases, patients can be discharged home with the pleural drainage, which can be subsequently removed in a specialist outpatient setting.
After pulmonary metastasectomy, the pulmonary function can be impaired in patients in treatment and also in long-term adult survivors, who often present restrictive syndrome and decreased physical functions (6,39). Survival outcomes vary depending on different histology (Table 1).
Surgery continues to be of paramount importance for metastatic and relapsing osteosarcoma patients and patients with metastatic soft tissues sarcomas, as well as in general for every histology patient whom are resistant to systemic treatment. Many authors have found that complete surgical resection of all sites of the disease has reported evidence to be a predictor of survival; moreover, repeated metastasectomy can improve survival or even cure some patients (4,7-9).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Scanagatta) for the series “Pediatric Thoracic Surgery” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.03.05). The series “Pediatric Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. PS serves as the unpaid Guest Editor of the series and an unpaid editorial board member of Pediatric Medicine from Jul 2018 to Jun 2020. PS reports personal fees from Baxter International, outside the submitted work. The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fuchs J, Seitz G, Handgretinger R, et al. Surgical treatment of lung metastases in patients with embryonal pediatric solid tumors: an update. Semin Pediatr Surg 2012;21:79-87. [Crossref] [PubMed]
- Predina JD, Puc MM, Bergey MR, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol 2011;6:913-9. [Crossref] [PubMed]
- Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther 2016;16:543-56. [Crossref] [PubMed]
- Temeck BK, Wexler LH, Steinberg SM, et al. Metastasectomy for Sarcomatous Pediatric Histologies: Results and Prognostic Factors. Ann Thorac Surg 1995;59:1385-9; discussion 1390. [Crossref] [PubMed]
- Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg 2006;41:194-9. [Crossref] [PubMed]
- Denbo JW, Zhu L, Srivastava D, et al. Long-term pulmonary function after metastasectomy for childhood osteosarcoma: a report from the St Jude lifetime cohort study. J Am Coll Surg 2014;219:265-71. [Crossref] [PubMed]
- Abel RM, Brown J, Moreland B, et al. Pulmonary metastasectomy for pediatric solid tumors. Pediatr Surg Int 2004;20:630-2. [Crossref] [PubMed]
- Scanagatta P, Girelli L. Metastasectomy in pediatric patients: indications, technical tips and outcomes. J Thorac Dis 2017;9:S1299-304. [Crossref] [PubMed]
- Meazza C, Scanagatta P, Luksch R, et al. How far can we go with surgery in metastatic osteosarcoma patients? Med Oncol 2015;32:223. [Crossref] [PubMed]
- Dillon P, Maurer H, Jenkins J, et al. A prospective study of nonrhabdomyosarcoma soft tissue sarcomas in the pediatric age group. J Pediatr Surg 1992;27:241-4; discussion 244-5. [Crossref] [PubMed]
- Pappo AS, Rao BN, Jenkins JJ, et al. Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 1999;33:76-82. [Crossref] [PubMed]
- Kayton ML, Meyers P, Wexler LH, et al. Clinical presentation, treatment, and outcome of alveolar soft part sarcoma in children, adolescents, and young adults. J Pediatr Surg 2006;41:187-93. [Crossref] [PubMed]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al. Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 2013;48:757-63. [Crossref] [PubMed]
- Carli M, Colombatti R, Oberlin O, et al. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. J Clin Oncol 2004;22:4787-94. Erratum in: J Clin Oncol 2005;23:248. [Crossref] [PubMed]
- Gulack BC, Rialon KL, Englum BR, et al. Factors associated with survival in pediatric adrenocortical carcinoma: An analysis of the National Cancer Data Base (NCDB). J Pediatr Surg 2016;51:172-7. [Crossref] [PubMed]
- Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol 1999;6:719-26. [Crossref] [PubMed]
- De León DD, Lange BJ, Walterhouse D, et al. Long-term (15 years) outcome in an infant with metastatic adrenocortical carcinoma. J Clin Endocrinol Metab 2002;87:4452-6. [Crossref] [PubMed]
- Dubois SG, London WB, Zhang Y, et al. Lung metastases in neuroblastoma at initial diagnosis: A report from the International Neuroblastoma Risk Group (INRG) project. Pediatr Blood Cancer 2008;51:589-92. [Crossref] [PubMed]
- Kammen BF, Matthay KK, Pacharn P, et al. Pulmonary metastases at diagnosis of neuroblastoma in pediatric patients: CT findings and prognosis. AJR Am J Roentgenol 2001;176:755-9. [Crossref] [PubMed]
- Briccoli A, Rocca M, Ferrari S, et al. Surgery for lung metastases in Ewing's sarcoma of bone. Eur J Surg Oncol 2004;30:63-7. [Crossref] [PubMed]
- Raciborska A, Bilska K, Rychłowska-Pruszyńska M, et al. Management and follow-up of Ewing sarcoma patients with isolated lung metastases. J Pediatr Surg 2016;51:1067-71. [Crossref] [PubMed]
- Letourneau PA, Shackett B, Xiao L, et al. Resection of pulmonary metastases in pediatric patients with Ewing sarcoma improves survival. J Pediatr Surg 2011;46:332-5. [Crossref] [PubMed]
- Uchiyama M, Iwafuchi M, Naito M, et al. A study of therapy for pediatric hepatoblastoma: prevention and treatment of pulmonary metastasis. Eur J Pediatr Surg 1999;9:142-5. [Crossref] [PubMed]
- Matsunaga T, Sasaki F, Ohira M, et al. Analysis of treatment outcome for children with recurrent or metastatic hepatoblastoma. Pediatr Surg Int 2003;19:142-6. [PubMed]
- Shi Y, Geller JI, Ma IT, et al. Relapsed hepatoblastoma confined to the lung is effectively treated with pulmonary metastasectomy. J Pediatr Surg 2016;51:525-9. [Crossref] [PubMed]
- Lange JM, Takashima JR, Peterson SM, et al. Breast cancer in female survivors of Wilms tumor: a report from the national Wilms tumor late effects study. Cancer 2014;120:3722-30. [Crossref] [PubMed]
- Green DM, Lange JM, Qu A, et al. Pulmonary disease after treatment for Wilms tumor: a report from the national wilms tumor long-term follow-up study. Pediatr Blood Cancer 2013;60:1721-6. [Crossref] [PubMed]
- Breslow NE, Lange JM, Friedman DL, et al. Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int J Cancer. 2010;127:657-66. [Crossref] [PubMed]
- Green DM, Breslow NE, Ii Y, et al. The role of surgical excision in the management of relapsed Wilms' tumor patients with pulmonary metastases: a report from the National Wilms' Tumor Study. J Pediatr Surg 1991;26:728-33. [Crossref] [PubMed]
- Dix DB, Seibel NL, Chi YY, et al. Treatment of Stage IV Favorable Histology Wilms Tumor With Lung Metastases: A Report From the Children's Oncology Group AREN0533 Study. J Clin Oncol 2018;36:1564-70. [Crossref] [PubMed]
- Cliffton EE, Pool JL. Treatment of lung metastases in children with combined therapy. Surgery and/or irradiation and chemotherapy. J Thorac Cardiovasc Surg 1967;54:403-21. [PubMed]
- Kilman JW, Kronenberg MW, O'Neill JA Jr, et al. Surgical resection for pulmonary metastases in children. Arch Surg 1969;99:158-65. [Crossref] [PubMed]
- Martini N, Huvos AG, Miké V, et al. Multiple pulmonary resections in the treatment of osteogenic sarcoma. Ann Thorac Surg 1971;12:271-80. [Crossref] [PubMed]
- van Geel AN, Pastorino U, Jauch KW, et al. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 1996;77:675-82. [Crossref] [PubMed]
- Durkovic S, Scanagatta P. Muscle-Sparing Thoracotomy: A Systematic Literature Review and the “AVE” Classification. J Surg Surgical Res 2015;1:35-44.
- Marta GM, Facciolo F, Ladegaard L, et al. Efficacy and safety of TachoSil® versus standard treatment of air leakage after pulmonary lobectomy. Eur J Cardiothorac Surg 2010;38:683-9. [Crossref] [PubMed]
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202. [Crossref] [PubMed]
- Erginel B, Gun Soysal F, Keskin E, et al. Pulmonary metastasectomy in pediatric patients. World J Surg Oncol 2016;14:27. [Crossref] [PubMed]
- Green DM, Zhu L, Wang M, et al. Pulmonary Function after Treatment for Childhood Cancer. A Report from the St. Jude Lifetime Cohort Study (SJLIFE). Ann Am Thorac Soc 2016;13:1575-85. [Crossref] [PubMed]
(English Language Editor: Jeremy Dean Chapnick, AME Publishing Company)
Cite this article as: Scanagatta P, Rolli L. Pulmonary metastasectomy in children and adolescents: a narrative review. Pediatr Med 2019;2:8.

