
儿童和青少年肺转移瘤切除术
近1/4患有实体瘤的儿童在最初诊断时有转移性疾病,另有20%在治疗期间或治疗后发生转移,最常见的转移部位在肺部[1]。手术在转移性疾病中的作用很大程度上取决于原发肿瘤的组织学类型。一般来说,辅助治疗困难的肿瘤最适合肺转移瘤切除术。
1 一般适应证
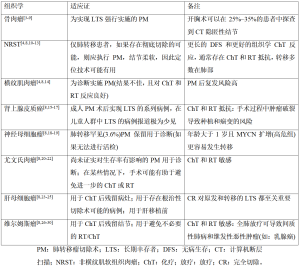
转移瘤切除术的作用在成人患者中已有积极研究,其将5年生存率从10%提高到了40%~55%[2]。然而,由于儿童实体瘤罕见,对儿童和青少年的研究较少,因此可以考虑手术的转移性肺部疾病的患者非常少见。在这些选定的病例中,手术指征评估应由多学科研究团队在选定的医院和机构进行。外科医生、儿科肿瘤学家、放射治疗师、心理学家和放射科医师以其不同的技能为肺转移瘤的管理做出贡献。肺转移瘤切除术的适应证因原始组织学类型不同而不同(表1)。
Full table
对于小儿转移性肿瘤,手术切除肺转移灶是一项耗时且要求高的手术治疗。从1950年代开始,出现了个案报道和少数案例的系列报道。1970年代病例系列显著增多,其中大多数包括不同类型的肿瘤[31-33],而胸部计算机断层扫描(computed tomography,CT)的普及和质量改进使得术前对肺结节的识别更加敏感(图1A)。在这种情况下,以小儿胸外科医生为主的多学科团队已经接受了儿童可以耐受单次、连续分期甚至再开胸术(在进一步复发后)的理念。使用非解剖性实质保留切除术(楔形切除术或精确切除术)会面临肺容量减少。转移性疾病患者的总生存率范围从20%到70%不等,具体取决于原发组织学(表1)。
2 技术方面
在准确的心肺评估后,患者将进行全身麻醉,并用双腔管插管以便实现术中单肺通气。
对于青少年来说,肺塌陷可以通过使用较小的双腔管来实现,这通常需要儿科支气管镜定位的辅助。对于年幼的孩子,传统的双腔管太大,最好放置支气管阻滞剂。阻滞剂在儿科支气管镜的直视下放置,以便进行选择性支气管排除。
完整的肺塌陷允许全肺触诊和胸部CT隐匿性结节的探查。据文献报道,为了达到彻底切除的目的,已经提出了不同的胸腔通路,从开胸手术到胸骨切开术再到电视辅助胸腔镜手术(video-assisted thoracoscopic surgery,VATS)[34]。
根据我们的经验,即使是在计划分期开胸术的情况下,通常首选的仍为微创、完整保留肌肉的横向开胸术[35]。
患者取侧卧位,在弓形或枕头上外展手臂,从而暴露侧胸壁。使用电灼或激光方法精确切除结节,以确保根治性手术有足够的边缘,同时保留周围的实质,与吻合器相比,这种方法所致的体积变形更为有限(图1B、1C)。实质缺损用3-0聚丙烯线进行单向或双向锁边缝合。多次切除时,在不损害术后肺功能和肺扩张的情况下封闭每一个缺损是不可能的。因此,在这些患者中,我们首选仅闭合深部的缺损而留下更浅表的缺损,用自体脂肪组织移植覆盖这些浅表的缺损,并使用经济有效的充气纤维蛋白胶或补丁来减少漏气[3,36-37]。
使用吻合器进行楔形切除术应用于少数的局部病变患者。在儿童中,吻合器的种类取决于肺实质和钉仓的厚度。其变化范围从用于外周结节的2.5 mm到用于更多中央楔形切除术或关闭中央支气管的3.5 mm不等。
解剖性切除术(肺段切除术、肺叶切除术或全肺切除术)应用于特定病例,因为只有在带来真正的肿瘤学优势的情况下才能证明肺大部切除术是合理的。
在存在胸膜沉积物的情况下,大多数儿科肿瘤不需要做胸膜切除术。当怀疑胸膜疾病时,应行术中冰冻活组织学检查进行评估,用以决定是否继续进行该手术。
术后疼痛控制可以使用硬膜外导管实现,该导管可以在麻醉诱导前放置,或者对于幼儿在手术结束时放置。如果硬膜外导管被拒绝或不可行,对有积极性和协作性的年龄较大的儿童和青少年,可以使用弹性泵或患者自控镇痛(patient controlled analgesia,PCA)进行静脉给药[35]。
转移瘤切除术的目的是完整切除所有结节。只有在初始切除后,术中评估不可手术的情况下,才允许部分减瘤。
应评估复发后的再次开胸手术。在这些情况下,应仔细规划手术干预,与先前的程序一起考虑当前复发的位置和特征。再次干预没有理论上的限制,因为可行性更多地取决于残余呼吸功能和疾病的自然病程,而不取决于技术难度。尽管如此,这些病例只能转诊到专业的肿瘤中心。
3 结论
围手术期病死率很少报道,而手术并发症的发生率在0%~12%之间变化[4-5,38],包括漏气、肺炎、呼吸功能不全和浅表伤口感染。
由于切除和缝合区域的医源性弹性缺乏,导致残余肺扩张减少,在这种情况下,唯一常见的问题是胸管的管理和呼吸物理治疗。在这些情况下,患者可以带胸腔引流出院回家,而胸腔引流随后可以在专科门诊取出。
肺转移瘤切除术后,接受治疗的患者以及长期成年幸存者会存在肺功能受损,这些患者经常出现限制性综合征和身体机能下降[6,39]。生存结果因不同的组织学而异(表1)。
对于转移性和复发性骨肉瘤患者、转移性软组织肉瘤患者以及对全身治疗有抵抗力的所有组织学患者来说,手术仍然是最重要的。许多作者发现,已有证据标明,对所有疾病部位进行完全手术切除是生存率的预测指标。此外,再次转移瘤切除术可以提高生存率,甚至治愈一些患者[4,7-9]。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Scanagatta) for the series “Pediatric Thoracic Surgery” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm.2019.03.05/coif). The series “Pediatric Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. PS serves as the unpaid Guest Editor of the series and an unpaid editorial board member of Pediatric Medicine from Jul 2018 to Jun 2020. PS reports personal fees from Baxter International, outside the submitted work. The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fuchs J, Seitz G, Handgretinger R, et al. Surgical treatment of lung metastases in patients with embryonal pediatric solid tumors: an update. Semin Pediatr Surg 2012;21:79-87. [Crossref] [PubMed]
- Predina JD, Puc MM, Bergey MR, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol 2011;6:913-9. [Crossref] [PubMed]
- Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther 2016;16:543-56. [Crossref] [PubMed]
- Temeck BK, Wexler LH, Steinberg SM, et al. Metastasectomy for Sarcomatous Pediatric Histologies: Results and Prognostic Factors. Ann Thorac Surg 1995;59:1385-9; discussion 1390. [Crossref] [PubMed]
- Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg 2006;41:194-9. [Crossref] [PubMed]
- Denbo JW, Zhu L, Srivastava D, et al. Long-term pulmonary function after metastasectomy for childhood osteosarcoma: a report from the St Jude lifetime cohort study. J Am Coll Surg 2014;219:265-71. [Crossref] [PubMed]
- Abel RM, Brown J, Moreland B, et al. Pulmonary metastasectomy for pediatric solid tumors. Pediatr Surg Int 2004;20:630-2. [Crossref] [PubMed]
- Scanagatta P, Girelli L. Metastasectomy in pediatric patients: indications, technical tips and outcomes. J Thorac Dis 2017;9:S1299-304. [Crossref] [PubMed]
- Meazza C, Scanagatta P, Luksch R, et al. How far can we go with surgery in metastatic osteosarcoma patients? Med Oncol 2015;32:223. [Crossref] [PubMed]
- Dillon P, Maurer H, Jenkins J, et al. A prospective study of nonrhabdomyosarcoma soft tissue sarcomas in the pediatric age group. J Pediatr Surg 1992;27:241-4; discussion 244-5. [Crossref] [PubMed]
- Pappo AS, Rao BN, Jenkins JJ, et al. Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 1999;33:76-82. [Crossref] [PubMed]
- Kayton ML, Meyers P, Wexler LH, et al. Clinical presentation, treatment, and outcome of alveolar soft part sarcoma in children, adolescents, and young adults. J Pediatr Surg 2006;41:187-93. [Crossref] [PubMed]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al. Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 2013;48:757-63. [Crossref] [PubMed]
- Carli M, Colombatti R, Oberlin O, et al. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. J Clin Oncol 2004;22:4787-94. Erratum in: J Clin Oncol 2005;23:248. [Crossref] [PubMed]
- Gulack BC, Rialon KL, Englum BR, et al. Factors associated with survival in pediatric adrenocortical carcinoma: An analysis of the National Cancer Data Base (NCDB). J Pediatr Surg 2016;51:172-7. [Crossref] [PubMed]
- Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol 1999;6:719-26. [Crossref] [PubMed]
- De León DD, Lange BJ, Walterhouse D, et al. Long-term (15 years) outcome in an infant with metastatic adrenocortical carcinoma. J Clin Endocrinol Metab 2002;87:4452-6. [Crossref] [PubMed]
- Dubois SG, London WB, Zhang Y, et al. Lung metastases in neuroblastoma at initial diagnosis: A report from the International Neuroblastoma Risk Group (INRG) project. Pediatr Blood Cancer 2008;51:589-92. [Crossref] [PubMed]
- Kammen BF, Matthay KK, Pacharn P, et al. Pulmonary metastases at diagnosis of neuroblastoma in pediatric patients: CT findings and prognosis. AJR Am J Roentgenol 2001;176:755-9. [Crossref] [PubMed]
- Briccoli A, Rocca M, Ferrari S, et al. Surgery for lung metastases in Ewing's sarcoma of bone. Eur J Surg Oncol 2004;30:63-7. [Crossref] [PubMed]
- Raciborska A, Bilska K, Rychłowska-Pruszyńska M, et al. Management and follow-up of Ewing sarcoma patients with isolated lung metastases. J Pediatr Surg 2016;51:1067-71. [Crossref] [PubMed]
- Letourneau PA, Shackett B, Xiao L, et al. Resection of pulmonary metastases in pediatric patients with Ewing sarcoma improves survival. J Pediatr Surg 2011;46:332-5. [Crossref] [PubMed]
- Uchiyama M, Iwafuchi M, Naito M, et al. A study of therapy for pediatric hepatoblastoma: prevention and treatment of pulmonary metastasis. Eur J Pediatr Surg 1999;9:142-5. [Crossref] [PubMed]
- Matsunaga T, Sasaki F, Ohira M, et al. Analysis of treatment outcome for children with recurrent or metastatic hepatoblastoma. Pediatr Surg Int 2003;19:142-6. [PubMed]
- Shi Y, Geller JI, Ma IT, et al. Relapsed hepatoblastoma confined to the lung is effectively treated with pulmonary metastasectomy. J Pediatr Surg 2016;51:525-9. [Crossref] [PubMed]
- Lange JM, Takashima JR, Peterson SM, et al. Breast cancer in female survivors of Wilms tumor: a report from the national Wilms tumor late effects study. Cancer 2014;120:3722-30. [Crossref] [PubMed]
- Green DM, Lange JM, Qu A, et al. Pulmonary disease after treatment for Wilms tumor: a report from the national wilms tumor long-term follow-up study. Pediatr Blood Cancer 2013;60:1721-6. [Crossref] [PubMed]
- Breslow NE, Lange JM, Friedman DL, et al. Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int J Cancer. 2010;127:657-66. [Crossref] [PubMed]
- Green DM, Breslow NE, Ii Y, et al. The role of surgical excision in the management of relapsed Wilms' tumor patients with pulmonary metastases: a report from the National Wilms' Tumor Study. J Pediatr Surg 1991;26:728-33. [Crossref] [PubMed]
- Dix DB, Seibel NL, Chi YY, et al. Treatment of Stage IV Favorable Histology Wilms Tumor With Lung Metastases: A Report From the Children's Oncology Group AREN0533 Study. J Clin Oncol 2018;36:1564-70. [Crossref] [PubMed]
- Cliffton EE, Pool JL. Treatment of lung metastases in children with combined therapy. Surgery and/or irradiation and chemotherapy. J Thorac Cardiovasc Surg 1967;54:403-21. [PubMed]
- Kilman JW, Kronenberg MW, O'Neill JA Jr, et al. Surgical resection for pulmonary metastases in children. Arch Surg 1969;99:158-65. [Crossref] [PubMed]
- Martini N, Huvos AG, Miké V, et al. Multiple pulmonary resections in the treatment of osteogenic sarcoma. Ann Thorac Surg 1971;12:271-80. [Crossref] [PubMed]
- van Geel AN, Pastorino U, Jauch KW, et al. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 1996;77:675-82. [Crossref] [PubMed]
- Durkovic S, Scanagatta P. Muscle-Sparing Thoracotomy: A Systematic Literature Review and the “AVE” Classification. J Surg Surgical Res 2015;1:35-44.
- Marta GM, Facciolo F, Ladegaard L, et al. Efficacy and safety of TachoSil® versus standard treatment of air leakage after pulmonary lobectomy. Eur J Cardiothorac Surg 2010;38:683-9. [Crossref] [PubMed]
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202. [Crossref] [PubMed]
- Erginel B, Gun Soysal F, Keskin E, et al. Pulmonary metastasectomy in pediatric patients. World J Surg Oncol 2016;14:27. [Crossref] [PubMed]
- Green DM, Zhu L, Wang M, et al. Pulmonary Function after Treatment for Childhood Cancer. A Report from the St. Jude Lifetime Cohort Study (SJLIFE). Ann Am Thorac Soc 2016;13:1575-85. [Crossref] [PubMed]
(English Language Editor: Jeremy Dean Chapnick, AME Publishing Company)

王彩虹。河北中石油中心医院。2002.09-2007.07就读于河北医科大学临床医学专业,2007.09-2010.07就读于北京大学医学部儿科学专业,研究方向免疫学,研究单位,首都儿科研究所。毕业后从事儿科临床工作至今。(更新时间:2021/7/14)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Scanagatta P, Rolli L. Pulmonary metastasectomy in children and adolescents . Pediatr Med 2019;2:8.


