
Comparison and culturing different types of cells from fresh breast milk with different culture medium
Introduction
Human breast milk is a complex liquid containing a large number of biological molecules and cellular components that not only provides nutrition for newborns but also facilitates immune regulation and tissue development (1). However, few studies investigated the properties of breast milk cells. With the development of new biotechnologies, researchers have demonstrated the essential roles of breast milk cells during breastfeeding (2-4).
Breast milk has heterogeneous populations including immunocytes and non-immune cells (5,6). Most of cells are immunocytes in the early stage of lactation, and then the number of immunocytes gradually reduced in the late stage of lactation (3,7). The flow cytometry determined that non-immune cells had been discovered gradually in breast milk (6,8,9). Early in the 1960s, mammary epithelial cells (MECs) in human breast milk with different cellular morphologies were found and identified as myoepithelial cells and luminal epithelial cells (10,11). Furthermore, Cregan et al. (12,13) found CK5 and NESTIN positive cells in human breast milk suggesting stem cell properties. In 2010, Satish et al. (14) found a population of cells which were positive for CD44, CD29, SCA-1 and negative for CD33, CD34, CD45 in breast milk. After being cultured with specific cocktails medium, these cells could differentiate into adipogenic, chondrogenic and osteogenic lineages. These results demonstrated that human breast milk contained mesenchymal stem cells (MSCs) (14-16). Furthermore, another study showed that breast milk had pluripotent stem cells with multilineage differentiation properties. These cells could express pluripotent markers including OCT4, SOX2, NANOG, and differentiate into three germ layers including neurons, cardiomyocytes, chondrocytes, and adipocytes (17-20).
There are three distinct stages of breast milk: colostrum, transitional milk and mature milk. Colostrum and transitional milk (C and T milk) usually refer to milk produced within two weeks after birth, and the mature milk is produced two weeks postpartum (8). Both C and T milk and mature milk have biological macromolecules and cells to provide nutritional ingredients for newborns. However, the difference in cellular components between C and T milk and mature milk have not been clearly investigated till now. In our study, we compared the differences in cellular properties between C and T milk and mature milk. Meanwhile, we studied the identities of different types of cells in breast milk, which could provide clues for studying specific cells from breast milk in the future.
Methods
Collection of breast milk samples
This study was approved by the Ethics Committee of the Children’s Hospital of Fudan University. Healthy breastfeeding women (n>50) were recruited, covering lactation stages from 2 days to 2 months. The C and T milk samples were collected from day 2 to day 7 post-delivery, and the mature milk were collected after two weeks. Breast milk samples were typically collected from volunteers in the morning through a sucker (2–200 mL), stored in a unique milk bottle, and transported to the laboratory on ice.
Breast milk cells isolation
Breast milk was diluted with equal volume of Dulbecco’s phosphate-buffered saline (DPBS) and centrifuged at 810 g for 20 minutes at room temperature. After centrifugation, the fat layer and liquid were discarded, and the cell pellet was washed three times with DPBS. Each centrifugation was performed at 300 g for 3 minutes. Finally, the cells were suspended in the corresponding medium. The total cell number and viability of each sample were determined with Countess II Automated Cell Counter (Thermo fisher) by Trypan Blue dye.
Cell viability in vitro
We tested the change of breast milk cell viability in vitro at four time points (2, 4, 6 and 8 hours). Before isolating the cells, the breast milk was stored at 4 °C. We collected same volume of breast milk every time point and the method of isolating cells was same as above described. The viability of cells was determined by the trypan blue dye on Countess II Automated Cell Counter (Thermo fisher).
Breast milk cell culture
Breast milk cells were cultured with M medium, F medium, and CM medium, respectively. The cells were seeded at density of 1×106 on each well of 6-well plate and incubated at 37 °C, 5% CO2, and 95% humidity. The M medium consisted of DMEM (Gibco), 10% fetal bovine serum (FBS, BI), 1× glutaMAX (Gibco), 1× MEM non-essential amino acid (NEAA, Gibco), and 1× antibiotic/antimycotic (Gibco). The F medium consisted of DMEM/F12 (Gibco), 10% FBS (BI), 1× glutaMAX (Gibco), 10 ng/mL epidermal growth factor (EGF, Peprotech), 5 µg/mL insulin (Sigma-Aldrich), 0.5 µg/mL hydrocortisone (Sigma-Aldrich), 10 ng/mL cholera toxin (Sigma-Aldrich), and 1× antibiotic/antimycotic (Gibco). The CM medium consisted of DMEM/F12 (Gibco), 20% knockout serum replacement (Gibco), 5 ng/mL basic fibroblast growth factor (bFGF, R&D), 1× glutaMAX (Gibco), 1× NEAA (Gibco), 100 µM 2-mercaptoethanol (Sigma-Aldrich), and 1× antibiotic/antimycotic (Gibco). The cells cultured in M medium or F medium were changed the medium everyday after day 4. Two weeks later, the cells were digested with 0.25% Trypsin-EDTA (Gibco) at 37 °C for 3 to 10 minutes and then passaged. When CM medium was used, the cells were seeded on feeder and the medium was changed everyday after day 3. Three weeks later, the cells were identified.
Immunofluorescence staining
The cells were fixed with 4% polyformaldehyde for 15 minutes, permeabilized with 0.2% Triton X-100 for 15 minutes and blocked with 10% donkey serum for 1 hour at room temperature. After blocking, the cells were incubated overnight at 4 °C with primary antibody. On the second day, the cells were washed with DPBS three times (10 minutes each time). The secondary antibody (Alexa Fluor 488 or 546, Invitrogen) was diluted with 5% donkey serum and incubated the cells for 1 hour at room temperature. Following three washes with DPBS, the slide was mounted, and the results were observed under a fluorescence microscope. Primary antibodies used were: mouse anti-CK14 (Abcam, 1:200), rabbit anti-CK18 (Abcam, 1:200), and goat anti-SOX2 (R&D, 1:500), rabbit anti-NANOG (Stemgent, 1:200).
Flow cytometry
Flow cytometry was mainly applied for the analysis of fresh breast milk and the cultured breast milk cells. The analyzed cell surface markers mainly included CD105 (BV421), CD90 (APC), CD73 (PE-CY7), CD45 (FITC), CD14 (PE), CD11c (APC), and CD44 (FITC) (all antibodies were bought from Becton Dickinson company). After isolation of fresh breast milk cells or the digestion of cultured cells, the cells were washed with DPBS for three times, followed by the incubation with diluted antibodies on ice for 30 minutes. Then, the cells were washed with DPBS three times. The acquisition of data was made with Flow Cytometer (Becton Dickinson), and data were analyzed by FlowJo software. The control of isotype group was set for all samples.
Statistical analyses
SPSS software was used for statistical analysis. In all studies, data were analyzed by independent t-test followed by Tukey’s multiple comparison tests. Statistical significance was determined at P<0.05.
Results
The characteristics of breast milk cells in C and T milk and mature milk
Breast milk samples were collected from healthy mothers, covering lactation stages from 2 days to 2 months. The result of cell density showed that the total cell concentration in breast milk decreased gradually during lactation stage. The total cell concentration in the breast milk were the highest on the second day after birth, and then decreased remarkably within first week (Figure 1A). Compared with the second day after birth (baseline), the total average cell concentration decreased by 20% (P=0.008) at the third day and 5% (P=0.014) at the fourth day. There was no significant change in total cell density from the second to the seventh week (Figure 1A). Therefore, C and T milk contained more cells than mature milk. It had been reported that the active ingredients of breast milk decreased when the milk was preserved in vitro for a while (21). However, it is unclear whether the decrease of cell viability differs between C and T milk and mature milk. In our study, cell viability was measured every 2 hours during 8 hours for C and T milk and mature milk in vitro. The results showed that the viability of breast milk cells in vitro decreased significantly every two hours (P<0.01), while there was no significant difference between C and T milk and mature milk (Figure 1B). This data suggested that although there were more cells in C and T milk than that in mature milk, yet there was no significant difference in cell viability between C and T milk and mature milk.
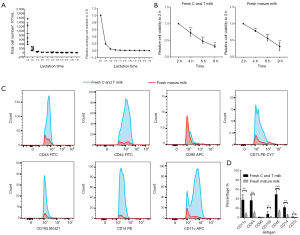
Previous studies found that breast milk contained different types of cells (5). In this study, we carried out flow cytometric characterization of various cell surface markers such as immune cells (CD14, CD11c, CD45), MSCs (CD90, CD105, CD73) and myoepithelial cells (CD44) in fresh breast milk. Our data indicated that all the expression of specific cell surface markers in C and T milk were significantly higher than that in mature milk, except for CD90 (Figure 1C,D). Especially, the immune cells significantly differed between C and T milk and mature milk. The positive rates of immune cell surface markers in C and T milk were 46.15%±6.42% for CD45, 36.75%±7.10% for CD11c and 21.30%±2.24% for CD14, while the ratio in the mature milk is 12.44%±2.01% for CD45, 8.30%±1.25% for CD11c, 4.82%±1.38% for CD14 (P<0.01 for each marker). However, positive markers for MSCs was relatively low, which were 8.52%±3.62% for CD105, 1.44%±0.07% for CD90, 1.64%±0.04% for CD73 in C and T milk and 1.46%±0.69% for CD105, 0.88%±0.21% for CD90, 0.73%±0.22% for CD73 in mature milk (P<0.05 for CD105 and CD73, P>0.05 for CD90) (Figure 1C,D). Moreover, the expression level of CD44 was 36.05%±5.99% in C and T milk and 10.07%±1.46% in mature milk (Figure 1C,D).
Immunocytes, MECs, and MSCs were identified in cultured breast milk cells with M medium
As data shown in Figure 1C,D, immunocytes and epithelial cells were the primary cells in both C and T milk and mature milk. In our further study, breast milk cells were cultured with three different media. The first medium called M medium which contained 10% FBS. The second medium contained 10% FBS and cytokines, and we called it F medium. The last one is suitable for cultural of pluripotent stem cells, which was called CM medium.
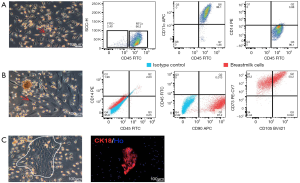
Human breast milk has a heterogeneous population of cells. In previous studies, the cells were mainly cultured in DMEM medium containing 10% FBS (14). In this study, breast milk cells were cultured in M medium. We found many adherent cells with three different cellular morphologies. Most cells were round with large nucleus (Figure 2A) while others were mesenchymal-like cells with long and thin cell body (Figure 2B) or colony growing cells with epithelial cells-like features (Figure 2C). By flow cytometry, we found that most cells with large nucleus (Figure 2A) were CD45-positive cells. Meanwhile, 98.1% of them were positive for CD11c (a marker of dendritic cells) and 3.88% were positive for CD14 (a monocyte marker), indicating these cells were mainly dendritic cells, and few of them were monocytes (Figure 2A). Further flow cytometric result showed that cells with mesenchymal-like morphology were negative for CD45 and CD14 but positive for CD90 (97.2%), CD73 (99.5%) and CD105 (50.2%) (Figure 2B). This data suggested that more than half of the cells with the mesenchymal-like morphology were MSCs. Finally, we used immunofluorescence to test cells with the epithelial cells-like morphology. Immunostaining showed clearly expression of CK18, a marker of luminal epithelial cells, in these cells (Figure 2C). The results showed that immunocytes, MSCs, and MECs appeared in breast milk cells after being cultured with M medium.
MECs and MSCs were identified in cultured breast milk cells with F medium
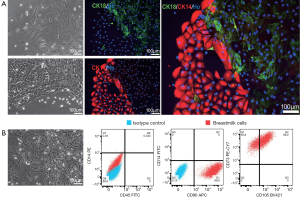
To investigate whether we could amplify a specific type of cells in breast milk, we tried to combine medium with cytokines to culture breast milk cells. After isolating the breast milk cells, the cells were cultured in F medium. We could see lots of adherent cells on fourth day after culturing. Three different morphologies (Figure 3A,B), i.e., spindle cells (Figure 3A, top), cobblestone-like cell colonies (Figure 3A, bottom), and mesenchymal-like cells (Figure 3B), were observed after ten days of culture. Immunofluorescence showed that Cytokeratin 14 (CK14, a myoepithelial cell marker) and CK18 were expressed in cells with Figure 3A morphologies, indicating both of the cells were MECs (Figure 3A). Furthermore, flow cytometry revealed that a large number of cells were positive for CD105 (69.2%), CD90 (99.8%) and CD73 (100%) in mesenchymal-like cells, while immunocyte markers CD45 and CD14 were negative. These data suggested that most of the mesenchymal-like cells were MSCs when breast milk was cultured with F medium (Figure 3B). Thus, MECs and MSCs were the primary cells when breast milk cells were cultured with F medium.
MECs and breast milk stem cells (BSCs) were identified in cultured breast milk cells with CM medium
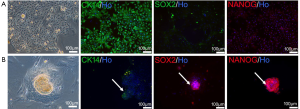
BSCs have been found in 2012 (17). To amplify these cells, we used CM medium to culture breast milk cells on feeder. Two major cell types were found with this medium. The morphologies were epithelioid cells (Figure 4A) or colony-like cells which were similar with human embryonic stem cells (hESCs). These colony-like cells were densely packed, with elevated clones in the middle (Figure 4B). Immunofluorescent staining showed that the first cell type was positive for the myoepithelial cell marker CK14 and negative for pluripotent stem cell markers NANOG and SOX2 (Figure 4A), indicating these cells were mainly epithelial cells. The second cell type was positive for NANOG and SOX2 and negative for CK14, suggesting that this type of cells had the characteristics of pluripotent stem cells. In summary, MECs and BSCs appeared when cultured breast milk cells with CM medium.
The efficiency of breast milk cell culture with different medium
The efficiency of breast milk cells cultured with different medium were compared between C and T milk and mature milk. As shown in Table 1, the M medium had higher efficiencies for immunocytes and epithelial cells. In 22 C and T milk samples, all can appear immunocytes and 21 samples could appear epithelial cells. While in 20 mature milk samples, 18 samples could get immunocytes and 15 samples could get epithelial cells. Only one sample was successfully cultured with MSCs in C and T milk and mature milk, respectively. Compared with M medium, F medium had a better efficiency in MECs culture, as MECs were obtained in all samples in C and T milk (20/20, 100%) and mature milk (20/20, 100%). However, the efficiency of MSCs were only 15% (3/20) in C and T milk and none (0/20) in mature milk. CM medium had high efficiency in both MECs and BSCs culture. 26/30 MECs were obtained in C and T milk and 22/22 in mature milk. While for BSCs, and 25/30 were obtained in C and T milk and 20/22 in mature milk.
Table 1
| Medium condition | Breast milk stage | Immune cell/total samples | MECs/total samples | MSCs/total samples | BSCs/total samples |
|---|---|---|---|---|---|
| M medium | C and T milk | 22/22 | 21/22 | 1/22 | No |
| Mature milk | 18/20 | 15/20 | 1/20 | No | |
| F medium | C and T milk | No | 20/20 | 3/20 | No |
| Mature milk | No | 20/20 | 0/20 | No | |
| CM medium | C and T milk | No | 26/30 | No | 25/30 |
| Mature milk | No | 22/22 | No | 20/22 |
MECs, mammary epithelial cells; MSCs, mesenchymal stem cells; BSCs; breast milk stem cells.
Discussion
There are many different types of cells in human breast milk, but how to obtain specific types of cells from breast milk become the crucial part for regenerative medicine and future treatment of different diseases. From the cytological perspective, we found that C and T milk had a higher cell concentration with more immunocytes and epithelial cells. In contrast, the mature milk had lower cells density. By comparing the characteristics of breast milk cells in different stages and culturing the breast milk cells with three different media, we obtained different proportions of adherent immunocytes, MECs, MSCs, and BSCs. This study provided new clues for culture of specific breast milk cells and disease treatment.
We reviewed previously published papers about breast milk cell culture (12,14,17), and made some modification while we culture cells from fresh breast milk. In this study, we could get different types of cells with M, F and CM medium. Although MSCs could be cultured in both M medium and F medium, the proportion was very low, which might be caused by low expression levels of these cells in fresh breast milk. Furthermore, the proliferation rate of MSCs cultured in F medium was faster than that in M medium (data not shown). In contrast, M medium was much better for culturing CD11c+ dendritic cells than F medium. From our Table 1, 95% (40/42) samples got dendritic cells with M medium, while 0 (0/40) samples got dendritic cells with F medium. The presence of these immune cells might affect the isolation and purification of MECs and MSCs due to the large proportion. Therefore, M medium was not recommended for amplification of MECs in breast milk.
As Hassiotou et al. (17) discovered BSCs in breast milk in 2012, BSCs as a potential source of regenerative medicine might be used for diseases treatment in the future. In our study, SOX2 and NANOG positive cells were obtained after culture of breast milk cells with CM medium. However, the amplification of these cells was still tricky (data not shown). Therefore, to obtain more BSCs, the cultural conditions need to be optimized in the further study.
As different medium can obtain different types of cells, how to separate target cells from the mixture became a significant challenge. In this study, the specific cells were separated from the mixture by different tolerance to trypsin. Compared with MSCs, epithelial cells were more tolerant to trypsin and took a longer time to be digested than MSCs. In this way, these two types of cells could be separated easily by different digestion time. After several passages, the purified MSCs and epithelial cells could be obtained.
In summary, C and T milk contains more cells than mature milk. A large number of epithelial cells can be obtained by culturing breast milk cells in F medium. Both F medium and M medium can be used for MSCs culture from breast milk. In contrast, the BSCs in breast milk can be cultured with CM medium.
Acknowledgments
Funding: This work was supported by the grant from Mount Sinai Hospital (Canada) to S Lee and grant 2017ZZ01008 from Shanghai Gene Therapy Project. We are grateful to all breastfeeding mothers who participated in this research.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.02.02). SL serves as an unpaid editorial board member of Pediatric Medicine from Jun 2018 to May 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Children’s Hospital of Fudan University (Approval ID: 2016/137). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr 2003;77:1537S-43S. [Crossref] [PubMed]
- Bode L, McGuire M, Rodriguez JM, et al. It’s Alive: Microbes and Cells in Human Milk and Their Potential Benefits to Mother and Infant. Adv Nutr 2014;5:571-3. [Crossref] [PubMed]
- Hassiotou F, Mobley A, Geddes DT, et al. Breastmilk imparts the mother’s stem cells to the infant: boosting early infant development? FASEB J 2015;29:876.
- Patki S, Patki U, Patil R, et al. Comparison of the levels of the growth factors in umbilical cord serum and human milk and its clinical significance. Cytokine 2012;59:305-8. [Crossref] [PubMed]
- Hassiotou F, Geddes DT, Hartmann PE. Cells in human milk: state of the science. J Hum Lact 2013;29:171-82. [Crossref] [PubMed]
- Witkowska-Zimny M, Kaminska-El-Hassan E. Cells of human breast milk. Cell Mol Biol Lett 2017;22:11. [Crossref] [PubMed]
- Trend S, de Jong E, Lloyd ML, et al. Leukocyte Populations in Human Preterm and Term Breast Milk Identified by Multicolour Flow Cytometry. PLoS One 2015;10:e0135580 [Crossref] [PubMed]
- Kaingade P. Breast milk cell components and its beneficial effects on neonates: need for breast milk cell banking. JPNIM 2017;6.
- Sani M, Hosseini SM, Salmannejad M, et al. Origins of the breast milk-derived cells; an endeavor to find the cell sources. Cell Biol Int 2015;39:611-8. [Crossref] [PubMed]
- Holmquist DG, Papanicolaou GN. The exfoliative cytology of the mammary gland during pregnancy and lactation. Ann NY Acad Sci 1956;63:1422-35. [Crossref] [PubMed]
- Saipin N, Noophun J, Chumyim P, et al. Goat milk: Non-invasive source for mammary epithelial cell isolation and in vitro culture. Anat Histol Embryol 2018;47:187-94. [Crossref] [PubMed]
- Cregan MD, Fan Y, Appelbee A, et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res 2007;329:129-36. [Crossref] [PubMed]
- Fan Y. Unravelling the Mystery of Stem/Progenitor Cells in Human Breast Milk. 2010;5:e14421.
- Patki S, Kadam S, Chandra V, et al. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell 2010;23:35-40. [Crossref] [PubMed]
- Abd Allah SH, Shalaby SM, El-Shal AS, et al. Breast milk MSCs: An explanation of tissue growth and maturation of offspring. IUBMB Life 2016;68:935-42. [Crossref] [PubMed]
- Sani M, Ebrahimi S, Aleahmad F, et al. Differentiation Potential of Breast Milk-Derived Mesenchymal Stem Cells into Hepatocyte-Like Cells. Tissue Eng Regen Med 2017;14:587-93. [Crossref] [PubMed]
- Hassiotou F, Beltran A, Chetwynd E, et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 2012;30:2164-74. [Crossref] [PubMed]
- Twigger AJ, Hodgetts S, Filgueira L, et al. From breast milk to brains: the potential of stem cells in human milk. J Hum Lact 2013;29:136-9. [Crossref] [PubMed]
- Hassiotou F, Hartmann PE. At the dawn of a new discovery: the potential of breast milk stem cells. Adv Nutr 2014;5:770-8. [Crossref] [PubMed]
- Hosseini SM, Talaei-Khozani T, Sani M, et al. Differentiation of human breast-milk stem cells to neural stem cells and neurons. Neurol Res Int 2014;2014:807896 [Crossref] [PubMed]
- Sousa SG, Delgadillo I, Saraiva JA. Human Milk Composition and Preservation: Evaluation of High-pressure Processing as a Nonthermal Pasteurization Technology. Crit Rev Food Sci Nutr 2016;56:1043-60. [Crossref] [PubMed]
Cite this article as: Tang C, Zhou Q, Lu C, Xiong M, Lee S. Comparison and culturing different types of cells from fresh breast milk with different culture medium. Pediatr Med 2019;2:5.

