
Impact of electronic cigarettes on pediatric, adolescent and young adult leukemia patients
Introduction
Electronic cigarettes are a form of inhaled nicotine delivery that operate on battery power, and their use is often called “vaping”. Originally promoted as a “safer” cigarette, the electronic cigarette industry has grown to a 22.4-billion-dollar global enterprise as of 2022. The United States Food and Drug Administration (US FDA) has offered some guidance on electronic cigarettes use, and recently banned one brand of these products (JUUL) (1). Due in large part to marketing efforts from electronic cigarette manufacturers, usage has increased amongst adolescent and young adult (AYA) populations, and this extends to childhood cancer patients and survivors (2). In fact, an increased rate of electronic cigarette use (>2-fold) in pediatric patients with a history of cancer than those without a history of cancer has been documented (3,4). One article documenting this trend examines survey data from the 2018 Behavioral Risk Factor Surveillance System to evaluate electronic cigarette use in 1,444 AYAs who have a history of cancer compared with 54,931 AYAs without cancer (3), and another article is a case study of AYAs who had an extensive history of using nicotine-delivery and was diagnosed with human papillomavirus-negative squamous cell carcinoma (5).
Of these published studies that investigated electronic cigarettes use in the pediatric cancer population, very few have focused on the health hazards relevant to late effects and survivorship, including effects on cardiotoxicity, neurotoxicity, carcinogenicity, and other sequelae of cancer treatment.
In the pediatric and AYA population, the most common type of cancer is a subset of leukemia. For the purpose of this review and consistent with the literature on AYA populations, we define pediatric patients as aged 1 day to 15 years, and AYA patients as aged 15 to 39 years (6). Leukemia, a disease caused by the production of abnormal leukocytes, is broadly classified by the cell of origin: myeloid or lymphoid (7). Leukemia is further subdivided into acute or chronic diseases depending on rate of proliferation. Types of leukemia include acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and chronic lymphocytic leukemia, with ALL being the most common pediatric leukemia. A few studies have investigated a possible link between tumorigenesis and electronic cigarettes but whether electronic cigarettes impart a risk of leukemogenesis remains unclear (5,8,9).
As pediatric cancer patients’ treatment outcomes improve and the population of childhood leukemia survivors increases (10), the long-term consequences and late effects of their treatments must be considered. Survivors of childhood ALL have a 1–6% increased risk of secondary malignancy associated with alkylating agents, anthracyclines, and etoposide; a 20–40% increased risk of neurocognitive effects associated with methotrexate, vincristine, steroids, or craniospinal radiation; a 36% increased risk of cardiotoxicity associated with a total cumulative anthracycline dose greater than 600 mg/m2, local radiation and obesity; and a 1–38% increased risk of endocrine abnormalities, including bone disorders, associated with steroids, radiation, high-dose methotrexate, mercaptopurine, calcium-poor diet, decreased physical activity, and obesity (11,12). These patients are also at risk for psychosocial disorders associated with the diagnosis of leukemia as well as its treatments (11). Prevention and treatment of these secondary effects are of vital importance.
Establishing the effects of electronic cigarettes on treatment efficacy for patients who use such devices before, during, and after leukemia treatment would enable clinicians to better counsel, advise and potentially treat this specific patient population. Furthermore, the long-term outcomes of electronic cigarettes are of particular importance to pediatric leukemia survivors as the population of survivors increase and already have an increased risk of morbidity and mortality. The average life expectancy of pediatric cancer survivors is already shorter compared to the general population by 14–30% depending on treatment (chemotherapy/radiation) (13-15). This review focuses on the trends in electronic cigarettes use, their clinical significance, and the long-term outcomes of electronic cigarette use in pediatric and AYA patients with leukemia and survivors of childhood leukemia. The purpose of this article is to summarize the potential harms of electronic cigarette use for pediatric cancer patients and survivors and to highlight topics for future research in understanding and mitigating risk of poor health outcomes from use of these products.
Electronic cigarettes
Electronic cigarettes, also known as “vapes”, “e-hookahs”, “vape pens”, and “electronic nicotine delivery systems”, can resemble traditional cigarettes, cigars, or pipes or everyday items such as USB flash drives or pens (16). Three different generations of electronic cigarettes are available with increasing sophistication in engineering that optimizes nicotine delivery. With the evolution of electronic cigarettes comes an increase in product variability, including chemical composition. In general, electronic cigarettes include a voltage-controlled battery that powers a heating element, such as heating coils, solders, and/or wicks, that aerosolizes a liquid component contained in a cartridge or pod. The aerosolized product, or vapor, is inhaled through a mouthpiece and then absorbed by the pulmonary alveoli and into systemic circulation. Newer devices add benzoic acid to nicotine which lowers the pH and allows protonated forms of nicotine to be easily inhaled (17).
The liquid component of an electronic cigarette contains nicotine but may also include other chemicals, including propylene glycol, volatile organic compounds (VOCs), flavoring compounds, and formaldehyde. A variety of electronic cigarettes contain tetrahydrocannabinol (THC) but this topic has been reviewed extensively elsewhere (18,19). Most electronic cigarettes do not contain tobacco, but because of their variable nicotine content, ranging from 3 to 48 mg/mL (20), are classified as tobacco products by the US FDA. However, the US FDA does not regulate the testing of all substances in electronic cigarettes, and it does not regulate the maximum total nicotine content electronic cigarettes can have. Given the large number of electronic cigarette products and the growing selection, there is a challenge for the US FDA to tightly regulate their production and composition, leading to inconsistencies in studies of their clinical significance.
Electronic cigarettes are fairly new to society but have been becoming increasingly popular. The first commercially developed electronic cigarette delivery system was invented in 2003 by Lik Hon, a Chinese pharmacist from Hong Kong (16). Contributing to their increasing popularity is a common belief that electronic cigarettes are less hazardous than traditional cigarettes (21). Many people who smoke traditional cigarettes use electronic cigarettes as smoking cessation aids (20); however, studies have shown that a majority of people who use electronic cigarettes as smoking cessation aids do not stop using electronic cigarettes after they quit smoking (22). In addition, emerging evidence suggests that AYAs who have never smoked traditional cigarettes are using electronic cigarettes. Thus far, there have been no electronic cigarette companies that have applied to the FDA Center for Drug and Evaluation Research to scientifically evaluate if electronic cigarettes are a successful smoking cessation tool (23). Therefore, it is clear that smoking cessation is not the only factor driving electronic cigarette use.
Electronic cigarettes present an overall public health concern, but recommendations for regulating their use lack consensus (23). In 2015, the American Association for Cancer Research (AACR) and the American Society of Clinical Oncology (ASCO) published a joint policy statement. The statement tried to advocate to decrease youth use of electronic cigarettes while remaining optimistic that electronic cigarettes could be a less harmful alternative to combustible tobacco cigarettes for adult smokers (24). An updated statement in 2022 from AACR and ASCO raised concern for increased youth use since the 2015 statement while evidence remains insufficient to show electronic cigarettes are more effective than current smoking cessation strategies (25). Many other public health organizations such as the United States Surgeon General, United States Preventative Services Task Force, and the National Comprehensive Cancer Network (NCCN) are demonstrating similar concern, highlighting the importance of electronic cigarette research to help guide recommendations in the general public as well as the pediatric oncology patient population.
Chemical composition of electronic cigarettes
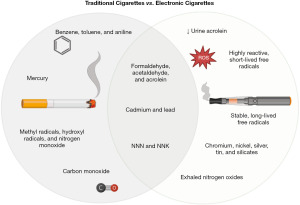
The clinical significance of electronic cigarettes may not be unlike that of conventional cigarettes (Table 1), as the two products both contain compounds linked to cancer; however, there are a few key differences. In order to understand the clinical significance of electronic cigarettes, we must first review traditional cigarettes.
Table 1
| Traditional cigarette combustion products | Examples | Effects on the body | Specific to electronic cigarettes |
|---|---|---|---|
| Carbonyl compounds | Formaldehyde*, acetaldehyde*, and acrolein* | Carcinogenic and cytotoxic | Lower levels of urine acrolein |
| Volatile organic compounds | Benzene, toluene, and aniline | Carcinogenic, hemotoxic, and neurotoxic | – |
| Tobacco-specific nitrosamines | NNN*, NNK* | Carcinogenic | – |
| Free radicals | Methyl radicals, hydroxyl radicals, and nitrogen monoxide | Cardiotoxic and neurotoxic | Highly reactive, short-lived free radicals and stable, long-lived free radicals |
| Metals | Cadmium*, lead*, and mercury | Carcinogenic, hemotoxic, neurotoxic, nephrotoxic | Chromium, nickel, silver, tin, and silicates |
| Gases | Carbon monoxide | – | Exhaled nitrogen oxide |
*, also found in electronic cigarettes. NNN, N-nitrosonornicotine; NNK, nicotine-derived nitrosamine ketone.
A complex mixture of combustion products, cigarette smoke can include more than 7,000 different chemicals and up to 1×1017 free radicals, including both reactive oxygen species (ROS) and reactive nitrogen species (20,26). Cigarette smoke has five important types of combustion products: carbonyl compounds, VOCs, tobacco-specific nitrosamines, free radicals, and metals (27,28). Carbonyl compounds include formaldehyde, acetaldehyde, and acrolein, which are carcinogenic and cytotoxic (27). VOCs include benzene, toluene, and aniline, which are carcinogenic, hemotoxic, and neurotoxic (27). Tobacco-specific nitrosamines include N-nitrosonornicotine (NNN) and nicotine-derived nitrosamine ketone (NNK), which are carcinogenic (27). Free radicals include methyl radicals, hydroxyl radicals, and nitrogen monoxide, which are cardiotoxic and neurotoxic (27). Metals include cadmium, lead, and mercury, which are carcinogenic, hemotoxic, neurotoxic, and nephrotoxic (27). Cigarette smoke also contains polycyclic aromatic compounds and toxic gases, including carbon monoxide (27).
Some of the chemical compounds in electronic cigarettes vapor are similar to those in cigarette smoke with few differences (Figure 1). Like cigarette smoke, electronic cigarette vapor can contain tobacco-derived contaminants, including tobacco-specific nitrosamines, such as NNN and NNK, and carbonyl compounds, such as formaldehyde, acetaldehydes, and acrolein (29). The total amount of aldehydes and acrolein generated by an electronic cigarette seems to be directly related to the battery voltage of the device (30,31). However, compared with traditional cigarettes, electronic cigarettes may result in lower levels of urine acrolein (31). Electronic cigarette vapor can have both highly reactive, short-lived free radicals and stable, long-lived free radicals (26) as well as low levels of metals, including cadmium, chromium, lead, nickel, silver, tin, and silicates, that are likely derived from the devices’ heating coils, solders, and/or wicks (32). Notably, because electronic cigarettes do not burn tobacco, their vapor does not contain carbon monoxide (20); however, they may result in higher levels of exhaled nitrogen oxide (33).
Trends in electronic cigarette use
The usage of electronic cigarettes in the AYA population has drastically increased over the past decade. In the 2012 US Surgeon General Report, approximately 1.78 million high school and middle school students nationwide reported that they had tried electronic cigarettes. Among these students who had ever used electronic cigarettes, only 9.3% reported never smoking conventional cigarettes (16) whereas 76% use both, indicating overlap in use of traditional and electronic cigarettes. In 2013, 13.1 million middle school and high school students were aware of electronic cigarettes (23,34). From the 2016 Surgeon General Report on electronic cigarettes, it was estimated that about 2.4 million high school students and around 600,000 middle school students had used an electronic cigarette at least once within the last 30 days (23). Data collected from 2011 to 2018 indicate that about 10 million adults (35,36) and more than 5 million middle and high school students in the United States use electronic cigarettes (36,37). In 2020 and 2021, approximately 8 out of 10 middle and high school students who use electronic cigarettes use a flavored version (38). These numbers continue to increase and emphasize the scope of the problem.
Based on National Youth Tobacco Survey data collected from 2011 to 2018, the rate of cigarette smoking among high school students was at a historic low (39). However, if both the use of cigarettes and that of electronic cigarettes are considered together, the number of adolescents using tobacco products is actually increasing (23). In the 2019 National Youth Tobacco Survey, the rate of current electronic cigarette use among high school students was estimated to be 27.5% [95% confidence interval (CI): 25.3%–29.7%] whereas the rate of traditional cigarette smoking was estimated to be 5.8% (95% CI: 4.6%–7.3%). From the same 2019 survey, an estimated 59.1% (95% CI: 54.8%–63.2%) of high school users and 54.1% (95% CI: 49.1%–59.0%) of middle school users reported JUUL as their usual electronic cigarette brand (37). In 2018, JUUL products comprised 75% of the electronic cigarette market (17). On June 23, 2022, the FDA made a press release that banned all JUUL products from the market pending court review. The recent action from the FDA validates public concerns about electronic cigarette use in all populations but more specifically the pediatric and AYA population.
A recent article from JAMA Netw Open in 2022 demonstrated an increased rate of first use of tobacco product by pediatric subjects increased from 28% in 2014 to 72% in 2021, and a median age of about 14 years old for first use of tobacco product (17). With specificity to electronic cigarettes, they noted a shift from “light use” to “heavier usage” where in 2014 less than 10% of all subjects used electronic cigarettes every single day in a month to almost 30% in 2021.
From 2014 to 2017, the prevalence of electronic cigarette uses among United States adults who reported a cancer diagnosis on the National Health Interview Survey increased from 8.5% to 10.7% (40). Using data from the 2018 Behavioral Risk Factor Surveillance System survey, Parsons et al. evaluated electronic cigarette use among AYAs (age 18–39 years) with or without a history of cancer (3). Of the 54,931 individuals surveyed, 1,444 reported a personal cancer history, although the survey did not specify which cancer subtype. Overall, the rate of past or current electronic cigarette use among AYAs with a history of cancer (46.7%) was significantly higher than among AYAs without a history of cancer (39.1%; P<0.001), and this was found across most demographic subgroups. In addition, the rate of current electronic cigarette use among AYAs with a history of cancer (31.3%) was higher than that among AYAs without a history of cancer (26.9%). While this difference was not significant (P=0.19), it still raises public concern.
The surveys and studies discussed above were analyses of self-reported data and thus subject to response/recall bias and may underestimate the scope of electronic cigarette use. However, it is clear that patients with a cancer diagnosis have an increased risk of electronic cigarette use. Overall, there is a gap in the literature defining electronic cigarette use in the pediatric and AYA population with leukemia.
Electronic cigarettes and the potential risk of leukemogenesis
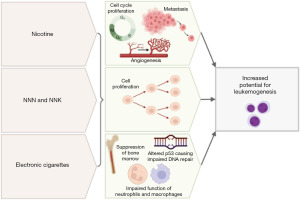
Understanding of the relationship between electronic cigarettes and the risk of leukemogenesis in pediatric and AYA patients is ongoing (Figure 2). Most of the current clinical recommendations are inferred by research involving traditional cigarettes, as well as ongoing research investigating the presence of carcinogens, DNA damage and inflammation in cell and animal culture cause by electronic cigarettes. Human data are very limited, and all of the human studies have relatively small total number of patient samples.
While electronic cigarette studies are limited, there is clear evidence to support a risk between traditional cigarettes and cancer. In a 2020 review by Miliano et al. (41), they reported that cigarette smoking contributed to 48.5% of deaths from 12 types of cancer, including AML and lung, oral cavity, oropharyngeal, esophagus, larynx, stomach, pancreas, colorectal, bladder, kidney, and cervical cancer. The greatest percentages of smoking-related deaths were not surprisingly from lung cancer (80.2%) and larynx cancer (76.6%) (42).
In 2014, Schaal et al. reported that nicotine can induce the cell-cycle progression, angiogenesis, and metastasis of lung and pancreatic cancers (43). They also found that tobacco-specific nitrosamines such as NNN and NNK increase cell proliferation, which can be a driving factor in leukemogenesis (44). This validates concern for nicotine-specific nitrosamines, whether from traditional cigarettes or electronic cigarettes, increasing the risk of leukemogenesis.
In 2021, Hamad et al. demonstrated that electronic cigarettes could alter the expression of the TP53 tumor suppressor gene (9). Their pilot study enrolled nine healthy non-smokers or former smokers without significant chronic medical conditions who were at least 18 ½ years old, had used electronic cigarettes at least 2 months prior to participation, and used electronic cigarettes at least eight times daily. Buccal and blood samples were obtained before and after electronic cigarette use (3 seconds of inhalation every 60 seconds, for a total of 20 inhalations over 20 minutes). Both buccal and blood samples showed the activation of pathways involved in DNA damage, DNA repair, the cell cycle, and cancer, including the mismatch DNA repair, nucleotide excision repair, base excision repair, homologous recombination, and non-homologous end joining pathways. TP53 expression was associated with greater inhalation volume and flow rate. Similar pathways have been implemented in the pathogenesis of leukemias, including AML and ALL (45,46). While this study describes another potential etiology of electronic cigarettes in terms of leukemogenesis, it only included adult patients and a very small sample size. Further studies are required to determine health effects in pediatric and AYA patients.
In 2016, Belver et al. demonstrated a correlation between smoking status and aberrations in hematopoietic stem cells and in myeloproliferative disorders driven by JAK2 mutations (e.g., JAK2V617F) (46), which suggests that smoking theoretically increases the risk of leukemia. Seeking to determine if electronic cigarettes convey a similar effect, Ramanathan et al. found that chronic exposure to electronic cigarette vapor (2 hours per day, 4 days per week, for 2 months) causes the suppression of bone marrow hematopoietic stem and progenitor cells in mice (36). They also found that, compared with control mice exposed to air alone, mice exposed to electronic cigarette vapor had fewer common c-Kit-positive myeloid progenitors and granulocyte-macrophage progenitors, both of which are important for hematopoiesis. Mutations or alterations in hematopoietic stem cells raise concern for clonal hematopoiesis, a premalignant condition caused by the clonal expansion of a blood cell with the same mutations often leading to cancers such as leukemia, lymphoma, or myeloma. These findings suggest that, similar to conventional cigarettes, electronic cigarettes can impair normal hematopoiesis in mice which may contribute to risk of leukemogenesis.
In 2019, Hickman et al. demonstrated that flavoring compounds from electronic cigarettes, including cinnamaldehyde, ethyl vanillin, and other aromatic aldehydes, decrease neutrophil oxidative burst and impair macrophage phagocytosis (47,48). The authors isolated neutrophils from the peripheral blood of healthy participants to measure the effects of different flavoring compounds on neutrophil oxidative burst. The authors also cultured neutrophils from healthy participants with increasing concentrations of flavoring compounds to measure phagocytosis and found impaired macrophage phagocytosis. They used a pHrodo-labeled bacterium, Staphylococcus aureus (S. aureus), that fluoresces upon phagocytosis in a pH-dependent manner to measure phagocytic capacity via flow cytometry. Flavoring compounds from electronic cigarettes had immunity-altering effects similar to those reported in a 2017 study by Clapp et al. (48), who used similar methods, including S. aureus bioparticles, to study phagocytosis in peripheral blood or bronchoalveolar lavage samples from healthy participants. These two studies demonstrate that the flavoring compounds in electronic cigarette vapor can alter cellular immunity and may be a potential mechanism of leukemogenesis.
Electronic cigarettes, late effects, and survivorship
The harmful effects of traditional cigarette use on the body have been well documented, and in the setting of a cancer diagnosis and throughout treatment appear to contribute to increased morbidity and mortality (8,11). For example, one study found that smoking was associated with an increased burden of treatment-related side effects in cancer patients compared to what was expected (49). In addition to increased adverse effects, a systemic review and meta-analysis found that treatment efficacy for several therapies, including radiation, chemoradiation, and certain targeted therapies, had reduced treatment efficacy in the patients who were smoking while receiving cancer treatment (50). Another review assessed the effect of tobacco smoke on radiation therapy outcomes and showed that the majority of studies indicate smoking is associated with a worse treatment outcome, including overall survival, progression free survival, and recurrence (50,51). While these studies were not done in the setting of pediatric and AYA leukemia, the results are relevant since the available treatment modalities are the same. Not only does smoking increase the risk of treatment-related adverse effects, but it can also negatively impact treatment efficacy/outcomes. Therefore, it is crucial for health providers to provide guidance on smoking cessation and the necessary resources for their patients to support them as they quit smoking. None of these studies have determined whether electronic cigarette use confers similarly poor outcomes. Since electronic cigarettes have been promoted as a smoking cessation strategy for traditional cigarettes in the past, it will be important to address this gap in knowledge.
The acute effects of electronic cigarettes are being investigated in ongoing studies, but the late effects of electronic cigarettes will likely remain unclear for some time. So far, the most concerning late effects appear to be related to cardiovascular disease and lung damage/disease. Preliminary studies show that chronic electronic cigarette use impairs endothelial function and is concerning for late cardiovascular damage (52). Furthermore, electronic cigarettes are known to cause lung injury and inflammation (53), which may increase the risk of late effect to the pulmonary system. In particular, these long-term consequences are concerning for childhood leukemia survivors, as these patients already have an increased risk of mortality due to the late effects secondary to cancer treatment (11).
To date, most of the cardiovascular effects of electronic cigarettes on humans are consistent with the known effects of nicotine (20,54). However, a few studies offer conflicting data and demonstrate less toxicity. Compared with cigarette smoke condensate, electronic cigarette vapor causes less oxidative stress (55). Farsalinos et al. investigated the viability of cardiomyoblasts exposed to electronic cigarette vapor or cigarette smoke (21), and discovered that electronic cigarette vapor is cytotoxic but significantly less cytotoxic than cigarette smoke. Although long-term data on the effects of electronic cigarettes on the heart are lacking, some preliminary data suggest that electronic cigarettes’ effects on the heart are less toxic than those of traditional cigarettes (21,55).
The acute cardiovascular effects of electronic cigarettes include heart failure characterized by decreased cardiac output, atherosclerosis, or coronary artery disease resulting in myocardial infarction (10). Free fatty acids, which are key elements in lipotoxicity, mitochondrial dysfunction, and cardiomyopathy can increase mitochondrial ROS (56-58), which are believed to be the main factors driving the cardiotoxic effects of electronic cigarettes. ROS are also an important mechanism by which conventional cigarette smoking induces cancer, cardiovascular disease, and chronic obstructive pulmonary disease (26). Chronic inflammation and insulin resistance may also contribute to cardiotoxicity from traditional cigarettes (16,59). Knowing that electronic cigarettes can cause an increase in ROS and inflammation to damage the heart, other organ systems are likely affected as well and may negatively impact survivorship.
The effects of electronic cigarettes on the pulmonary system are also of particular importance, as leukemia patients already have an increased risk of morbidity secondary to the pulmonary effects of the disease, its treatments, and a weakened immune system (60). In one study using a murine model, the pulmonary effects of electronic cigarettes included reduced mucociliary clearance, increased inflammation, and increased ROS (61). In a 2015 study by Sussan et al., mouse models had increased levels of oxidative stress markers, such as malondialdehyde and lipid peroxidation, after exposure to electronic cigarette vapor (62). Another study demonstrated that bronchiolar lavage samples from mice exposed to electronic cigarette vapor had increased numbers of macrophages and other infiltrating inflammatory cells (63), similar to the findings of Clapp et al. (48). Another study suggested the accumulation of insoluble agents from electronic cigarette vapor in the airways may cause decreased mucociliary clearance (64), which Sussan et al. found to increase the risk of bacterial, fungal, and viral infection in their mouse model (62). Alterations of redox homeostasis are alarming as ROS is an important player in tumorigenesis and cancer cells are typically characterized by high concentrations of ROS (65). Its involvement in tumorigenesis is multi-factorial as ROS can activate oncogenic signaling pathways, cause alterations of the DNA, reshape the tumor microenvironment, and contribute to angiogenesis (66). Therefore, increased ROS levels are a plausible etiology for leukemogenesis related to electronic cigarette use. Beyond changes in ROS, pulmonary studies of electronic cigarette use suggest that alterations in immunity may also contribute to tumor development.
Only a few studies have investigated the pulmonary effects of electronic cigarettes in humans. In 2013, Flouris et al. reported that active exposure to electronic cigarette vapor is not associated with any significant impairment in lung function (as measured by spirometry) or with decreased levels of serum cotinine, exhaled carbon monoxide, or exhaled nitric oxide (67). However, the study included only 30 patients, and it did not assess the long-term effects of exposure or the effect of the dose. Data on the pulmonary effects of long-term or secondhand electronic cigarette exposure in humans (68), which are of particular importance to cancer survivors, are exceedingly limited.
Conclusions
There is growing support in the current body of literature that suggests possible etiology of pediatric leukemogenesis secondary to electronic cigarette use, including aberrations in cell cycle, DNA repair, production of ROS, impaired hematopoiesis and altered immunity. However, these studies are primarily in cell culture and animal models or in human studies with small number of subjects. There is a need for long-term human studies to determine effects of electronic cigarettes before, during and after treatment in terms of treatment efficacy. It will take many years, possibly decades, to identify the effects of electronic cigarettes on childhood leukemia survivors. Whether electronic cigarettes are less harmful to humans than traditional cigarettes remains unclear. The studies reviewed herein all had limitations, and none included patients with leukemia nor specifically patients in the pediatric and AYA population. Future studies are needed to determine epidemiologic patterns of electronic cigarette use in pediatric and AYA patients, health effects, toxicity, environmental effects, and psychological effects. Such studies could guide the regulation of electronic cigarettes and inform general health providers on how to counsel pediatric and AYA patients with leukemia. Until definitive data are available, clinicians should strongly advise pediatric and AYA patients with leukemia to avoid using electronic cigarettes given the scientific evidence reviewed in this article and its plausible risk to negatively impact survivorship.
Acknowledgments
We acknowledge and appreciate the assistance of Joseph Munch in the Research Medical Library at the University of Texas MD Anderson Cancer Center.
Funding: This work was supported by the Cancer Prevention and Research Institute of Texas (CPRIT), the Texas Tobacco Settlement – Molecular Mechanisms of Tobacco Carcinogenesis Pilot Project Grant awarded (No. RP200381 to J.C.), and by the Training Core of the Clinical Translational Science (CTSA) Award to UTHealth Houston (No. TL1 TR003169 to L.M.S.).
Footnote
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-23-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-23-43/coif). J.C. serves as an unpaid editorial board member of Pediatric Medicine from May 2022 to April 2024. J.C. also received Grants from Texas Tobacco Settlement – Molecular Mechanisms of Tobacco Carcinogenesis (No. RP200381). L.M.S. received grants from UTHealth Houston Center for Clinical and Translational Sciences TL1 Program (No. TL1 TR003169). The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Omaiye EE, Luo W, McWhirter KJ, et al. Disposable Puff Bar Electronic Cigarettes: Chemical Composition and Toxicity of E-liquids and a Synthetic Coolant. Chem Res Toxicol 2022;35:1344-58. [Crossref] [PubMed]
- Jones K, Salzman GA. The Vaping Epidemic in Adolescents. Mo Med 2020;117:56-8. [PubMed]
- Parsons HM, Jewett PI, Sadak K, et al. e-Cigarette Use Among Young Adult Cancer Survivors Relative to the US Population. JAMA Oncol 2020;6:923-6. [Crossref] [PubMed]
- Shelton CM, Black H, Proctor J, et al. A Comprehensive Review of Vaping Use in Pediatric Patients and Recent Changes in Regulatory Laws. J Pediatr Pharmacol Ther 2022;27:109-19. [Crossref] [PubMed]
- Klawinski D, Hanna I, Breslin NK, et al. Vaping the Venom: Oral Cavity Cancer in a Young Adult With Extensive Electronic Cigarette Use. Pediatrics 2021;147:e2020022301. [Crossref] [PubMed]
- Creutzig U, Kutny MA, Barr R, et al. Acute myelogenous leukemia in adolescents and young adults. Pediatr Blood Cancer 2018;65:e27089. [Crossref] [PubMed]
- Chennamadhavuni A, Lyengar V, Mukkamalla SKR, et al. Leukemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Mravec B, Tibensky M, Horvathova L, et al. E-Cigarettes and Cancer Risk. Cancer Prev Res (Phila) 2020;13:137-44. [Crossref] [PubMed]
- Hamad SH, Brinkman MC, Tsai YH, et al. Pilot Study to Detect Genes Involved in DNA Damage and Cancer in Humans: Potential Biomarkers of Exposure to E-Cigarette Aerosols. Genes (Basel) 2021;12:448. [Crossref] [PubMed]
- Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 2010;28:2625-34. [Crossref] [PubMed]
- Kızılocak H, Okcu F. Late Effects of Therapy in Childhood Acute Lymphoblastic Leukemia Survivors. Turk J Haematol 2019;36:1-11. [Crossref] [PubMed]
- Espinoza-Derout J, Hasan KM, Shao XM, et al. Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice. Am J Physiol Heart Circ Physiol 2019;317:H445-59. [Crossref] [PubMed]
- Yeh JM, Ward ZJ, Chaudhry A, et al. Life Expectancy of Adult Survivors of Childhood Cancer Over 3 Decades. JAMA Oncol 2020;6:350-7. [Crossref] [PubMed]
- Cupit-Link MC, Kirkland JL, Ness KK, et al. Biology of premature ageing in survivors of cancer. ESMO Open 2017;2:e000250. [Crossref] [PubMed]
- Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J 2011;1:e16. [Crossref] [PubMed]
- Bhatnagar A, Whitsel LP, Ribisl KM, et al. Electronic cigarettes: a policy statement from the American Heart Association. Circulation 2014;130:1418-36. [Crossref] [PubMed]
- Glantz S, Jeffers A, Winickoff JP. Nicotine Addiction and Intensity of e-Cigarette Use by Adolescents in the US, 2014 to 2021. JAMA Netw Open 2022;5:e2240671. [Crossref] [PubMed]
- Chadi N, Minato C, Stanwick R. Cannabis vaping: Understanding the health risks of a rapidly emerging trend. Paediatr Child Health 2020;25:S16-20. [Crossref] [PubMed]
- Civiletto CW, Hutchison J. Electronic Vaping Delivery of Cannabis and Nicotine. In: StatPearls. Treasure Island (FL): StatPearls Publishing; September 19, 2022.
- Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol 2017;14:447-56. [Crossref] [PubMed]
- Farsalinos KE, Romagna G, Allifranchini E, et al. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health 2013;10:5146-62. [Crossref] [PubMed]
- Marczylo T. How bad are e-cigarettes? What can we learn from animal exposure models? J Physiol 2020;598:5073-89. [Crossref] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General [Internet]. Atlanta (GA): Centers for Disease Control and Prevention (US); 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538680/
- Brandon TH, Goniewicz ML, Hanna NH, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. Clin Cancer Res 2015;21:514-25. [Crossref] [PubMed]
- Herbst RS, Hatsukami D, Acton D, et al. Electronic Nicotine Delivery Systems: An Updated Policy Statement From the American Association for Cancer Research and the American Society of Clinical Oncology. J Clin Oncol 2022;40:4144-55. [Crossref] [PubMed]
- Goel R, Durand E, Trushin N, et al. Highly reactive free radicals in electronic cigarette aerosols. Chem Res Toxicol 2015;28:1675-7. [Crossref] [PubMed]
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23:133-9. [Crossref] [PubMed]
- Smith CJ, Livingston SD, Doolittle DJ. An international literature survey of "IARC Group I carcinogens" reported in mainstream cigarette smoke. Food Chem Toxicol 1997;35:1107-30. [Crossref] [PubMed]
- Lisko JG, Tran H, Stanfill SB, et al. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob Res 2015;17:1270-8. [Crossref] [PubMed]
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014;16:1319-26. [Crossref] [PubMed]
- McRobbie H, Phillips A, Goniewicz ML, et al. Effects of Switching to Electronic Cigarettes with and without Concurrent Smoking on Exposure to Nicotine, Carbon Monoxide, and Acrolein. Cancer Prev Res (Phila) 2015;8:873-8. [Crossref] [PubMed]
- Williams M, Villarreal A, Bozhilov K, et al. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 2013;8:e57987. [Crossref] [PubMed]
- Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health 2014;217:628-37. [Crossref] [PubMed]
- Wang B, King BA, Corey CG, et al. Awareness and use of non-conventional tobacco products among U.S. students, 2012. Am J Prev Med 2014;47:S36-52. [Crossref] [PubMed]
- Mirbolouk M, Charkhchi P, Kianoush S, et al. Prevalence and Distribution of E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016. Ann Intern Med 2018;169:429-38. [Crossref] [PubMed]
- Ramanathan G, Craver-Hoover B, Arechavala RJ, et al. E-Cigarette Exposure Decreases Bone Marrow Hematopoietic Progenitor Cells. Cancers (Basel) 2020;12:2292. [Crossref] [PubMed]
- Cullen KA, Gentzke AS, Sawdey MD, et al. e-Cigarette Use Among Youth in the United States, 2019. JAMA 2019;322:2095-103. [Crossref] [PubMed]
- Cooper M, Park-Lee E, Ren C, et al. Notes from the Field: E-cigarette Use Among Middle and High School Students - United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1283-5. [Crossref] [PubMed]
- Gentzke AS, Creamer M, Cullen KA, et al. Vital Signs: Tobacco Product Use Among Middle and High School Students - United States, 2011-2018. MMWR Morb Mortal Wkly Rep 2019;68:157-64. [Crossref] [PubMed]
- Sanford NN, Sher DJ, Xu X, et al. Trends in Smoking and e-Cigarette Use Among US Patients With Cancer, 2014-2017. JAMA Oncol 2019;5:426-8. [Crossref] [PubMed]
- Miliano C, Scott ER, Murdaugh LB, et al. Modeling drug exposure in rodents using e-cigarettes and other electronic nicotine delivery systems. J Neurosci Methods 2020;330:108458. [Crossref] [PubMed]
- Peerschke EI, Ghebrehiwet B. cC1qR/CR and gC1qR/p33: observations in cancer. Mol Immunol 2014;61:100-9. [Crossref] [PubMed]
- Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res 2014;12:14-23. [Crossref] [PubMed]
- Irons RD, Stillman WS. Cell proliferation and differentiation in chemical leukemogenesis. Stem Cells 1993;11:235-42. [Crossref] [PubMed]
- Licht JD, Sternberg DW. The molecular pathology of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program 2005;137-42. [Crossref] [PubMed]
- Belver L, Ferrando A. The genetics and mechanisms of T cell acute lymphoblastic leukaemia. Nat Rev Cancer 2016;16:494-507. [Crossref] [PubMed]
- Hickman E, Herrera CA, Jaspers I. Common E-Cigarette Flavoring Chemicals Impair Neutrophil Phagocytosis and Oxidative Burst. Chem Res Toxicol 2019;32:982-5. [Crossref] [PubMed]
- Clapp PW, Pawlak EA, Lackey JT, et al. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol 2017;313:L278-92. [Crossref] [PubMed]
- Peppone LJ, Mustian KM, Morrow GR, et al. The effect of cigarette smoking on cancer treatment-related side effects. Oncologist 2011;16:1784-92. [Crossref] [PubMed]
- Bergman M, Fountoukidis G, Smith D, et al. Effect of Smoking on Treatment Efficacy and Toxicity in Patients with Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) 2022;14:4117. [Crossref] [PubMed]
- Perdyan A, Jassem J. Impact of Tobacco Smoking on Outcomes of Radiotherapy: A Narrative Review. Curr Oncol 2022;29:2284-300. [Crossref] [PubMed]
- Mohammadi L, Han DD, Xu F, et al. Chronic E-Cigarette Use Impairs Endothelial Function on the Physiological and Cellular Levels. Arterioscler Thromb Vasc Biol 2022;42:1333-50. [Crossref] [PubMed]
- Park JA, Crotty Alexander LE, Christiani DC. Vaping and Lung Inflammation and Injury. Annu Rev Physiol 2022;84:611-29. [Crossref] [PubMed]
- Basma H, Tatineni S, Dhar K, et al. Electronic cigarette extract induced toxic effect in iPS-derived cardiomyocytes. BMC Cardiovasc Disord 2020;20:357. [Crossref] [PubMed]
- Czekala L, Simms L, Stevenson M, et al. High Content Screening in NHBE cells shows significantly reduced biological activity of flavoured e-liquids, when compared to cigarette smoke condensate. Toxicol In Vitro 2019;58:86-96. [Crossref] [PubMed]
- Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 2011;18:139-43. [Crossref] [PubMed]
- Chong CR, Clarke K, Levelt E. Metabolic Remodeling in Diabetic Cardiomyopathy. Cardiovasc Res 2017;113:422-30. [Crossref] [PubMed]
- Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 2008;45:231-41. [Crossref] [PubMed]
- Wang Z, Wang D, Wang Y. Cigarette Smoking and Adipose Tissue: The Emerging Role in Progression of Atherosclerosis. Mediators Inflamm 2017;2017:3102737. [Crossref] [PubMed]
- Garcia JB, Lei X, Wierda W, et al. Pneumonia during remission induction chemotherapy in patients with acute leukemia. Ann Am Thorac Soc 2013;10:432-40. [Crossref] [PubMed]
- Laube BL, Afshar-Mohajer N, Koehler K, et al. Acute and chronic in vivo effects of exposure to nicotine and propylene glycol from an E-cigarette on mucociliary clearance in a murine model. Inhal Toxicol 2017;29:197-205. [Crossref] [PubMed]
- Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 2015;10:e0116861. [Crossref] [PubMed]
- Glynos C, Bibli SI, Katsaounou P, et al. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol 2018;315:L662-72. [Crossref] [PubMed]
- Randell SH, Boucher RC. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 2006;35:20-8. [Crossref] [PubMed]
- Xing F, Hu Q, Qin Y, et al. The Relationship of Redox With Hallmarks of Cancer: The Importance of Homeostasis and Context. Front Oncol 2022;12:862743. [Crossref] [PubMed]
- Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci 2020;77:4459-83. [Crossref] [PubMed]
- Flouris AD, Chorti MS, Poulianiti KP, et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol 2013;25:91-101. [Crossref] [PubMed]
- Collaco JM, Drummond MB, McGrath-Morrow SA. Electronic cigarette use and exposure in the pediatric population. JAMA Pediatr 2015;169:177-82. [Crossref] [PubMed]
Cite this article as: Sarkar S, Stitzlein LM, Chandra J. Impact of electronic cigarettes on pediatric, adolescent and young adult leukemia patients. Pediatr Med 2024;7:3.

