
The leaky gut: a narrative review on the role of epithelial cell permeability in necrotizing enterocolitis
Introduction
Background
Necrotizing enterocolitis (NEC) is a devastating diagnosis involving significant intestinal injury that typically affects premature neonates. Despite advancements in neonatal care in recent years, NEC remains the leading cause of morbidity and mortality in premature infants, affecting about 2–5% of all premature infants and approximately 7% in very low birth weight premature infants (1,2). The overall mortality is most severe among infants requiring surgery, however on average, ranges between 20% and 30%, but approaches 100% in infants with the most severe form of the disease (1,2).
NEC is a complex diagnosis and has a range of risk factors and cellular processes that contribute to disease susceptibility and its pathogenesis. Despite years of active research, the precise mechanisms underlying the intestinal injury remain to be fully elucidated. Consistent risk factors for NEC intestinal injury include low birth weight, prematurity, intestinal immaturity including intestinal barrier function and inflammation, microbial colonization, and history of enteral feeding, in particular with high osmotic formula or a rapid advancement in feeding (Figure 1) (1,3,4). Other factors such as gastric acid suppression, circulatory disorders, hypoxia, and a poor intrauterine environment have been postulated to contribute to NEC, however these data are inconclusive (1-3).
The intestinal injury seen in NEC is associated with a hyperinflammatory response and dysregulation of the normal cellular processes crucial to intestinal barrier function (1,5). The semi-permeable intestinal barrier helps to regulate microbiome homeostasis and has been a focus of research not only in the diagnosis of NEC, but also in inflammatory bowel disease (IBD) signifying its importance in the pathology of intestinal injury (6,7). A healthy intestinal barrier is a dynamic system with cells interacting with the gut microbiome, the immune system, and chemical signals. The mainstay of the intestinal barrier is the intestinal epithelial cells which consist of enterocytes, goblet cells, enteroendocrine cells, and Paneth cells (6,8). Microbial gut dysbiosis occurring through insults such as initiation of enteral feeds, intestinal ischemia, or pathologic bacterial translocation can perpetuate intestinal barrier injury such as that seen in NEC or IBD (9,10).
Rationale and knowledge gap
The premature infant gut has many differences when compared to full term infants or adults (11). The microbiome seen in premature neonates has been shown to be less diverse than in full term infants leading to alterations in the interactions between the intestinal epithelial cells and the components they produce (6-8,11-13). The microbial dysbiosis that contributes to intestinal barrier disruption that can be seen in premature infants has been hypothesized to be attributed to abnormal peristalsis, low gastric acid production, altered intestinal mucin layer, and altered intestinal enzyme activities (14-17). There is a paucity of knowledge regarding the intestinal epithelial cell barrier in regards to causality, prevention, and potential treatments for intestinal injury seen in NEC.
Objective
We aim to produce this narrative review focusing on the intestinal epithelial cell barrier and the role of increased permeability in the intestinal injury seen in NEC along with current and potential future research strategies on the subject. We present this article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-22-41/rc).
Methods
A literature review using the databases PubMed and Google Scholar was performed utilizing search terms “necrotizing enterocolitis”, “epithelial cell permeability in NEC”, “inflammatory reaction in NEC”, “tight junctions in NEC”, “gastric motility in NEC”, “intestinal prematurity in NEC” (Table 1). This search was performed from July 2022 to August 2022 without a specified time frame.
Table 1
| Items | Specification |
|---|---|
| Date of search | 07/2022–08/2022 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used | Necrotizing enterocolitis; epithelial cell permeability in NEC; inflammatory reaction in NEC; tight junctions in NEC; gastric motility in NEC; intestinal prematurity in NEC |
| Timeframe | Literature published until search date |
| Inclusion and exclusion criteria | Inclusion: English language, focused on the above search terms in relation to necrotizing enterocolitis |
| Exclusion: involving non-specific inflammatory bowel disease | |
| Selection process | Selection of references was agreed upon all authors |
NEC, necrotizing enterocolitis.
Discussion
The intestinal barrier
The semi-permeable intestinal barrier consists of the outer mucus layer, the intestinal epithelial cells, and the inner lamina propria (18). The outer mucus layer is mainly formed from mucin 1 and 2, secreted by goblet cells, resulting in an adherent structure overlying the intestinal epithelium (7,13,19). Its main purpose is to help prevent bacterial translocation and invasion by altering adhesion and movement to the intestinal barrier (19,20). The mucus layer changes with age, in response to bacteria, and following injury (21-23). Alterations in the mucus layer have mainly been demonstrated in animal studies showing that it is decreased in prematurity allowing larger, potentially pathogenic molecules to come into contact with the underlying intestinal epithelium (21-23). The premature neonate intestine has been shown to express lower levels of mucin 1 and 2 therefore decreasing the defense mechanisms produced by mucin 1 and the viscosity of the mucus layer produced by mucin 2 (19,20). This allows for easier bacterial translocation to the underlying intestinal epithelium resulting in an inflammatory response further perpetuating intestinal injury (19,20,22). Once able to traverse through the outer mucus layer of the intestinal barrier, pathogens encounter another physical barrier, the intestinal epithelial cells (24). These consist of four major cell types: the enterocytes, goblet cells, Paneth cells and enteroendocrine cells (6-8). Intercellular junctions mediate the interactions between the four major types of cells allowing for regulation of the semi-permeable nature present in intestinal epithelial cell barrier (6,7). An intact epithelial cell layer with properly functioning intercellular junctions is crucial in maintaining homeostasis and protecting against potential infection in conjunction with other components of the intestinal system (1,25).
Intercellular junctions
Intercellular junctions consist of tight junctions, adherence junctions, and desmosomes (6,7). Tight junctions are continuous, circumferential structures that contribute to the barrier at the apical end of the intercellular spaces made up of occludin, ZO-1, cingulin, and claudin proteins (6). This serves to maintain the polarity of cells and preserve the distinct environment on either side of the barrier while maintaining a semi-permeable intestinal epithelial cell layer (6). They are very adaptable and responsible for regulating the transport of nutrients and preventing leakage of macromolecules from the lumen (6). In both human and animal studies, it has been demonstrated that in NEC diagnoses the genes responsible for coding the proteins claudin-4, ZO-1, and occludin were severely downregulated compared to controls altering the integrity of the tight junctions and intestinal epithelium (26). To further study this phenomenon enteroids, an intestinal organoid that is a self-organizing group of stem cells consisting of all four epithelial cell types, are generated and can be subjected to conditions to induce NEC by administering lipopolysaccharide (LPS) and hypoxia. Following this treatment to induce NEC, the enteroids have been shown to exhibit alterations in tight junction proteins and have a heightened inflammatory response altering intestinal barrier function compared to controls (19). Interactions with LPS have been shown to down regulate tight junction protein gene expression and through studies on IBD it has been demonstrated that inflammatory cytokines can also alter tight junctions (7). Tumor necrosis factor-α (TNF-α), an inflammatory cytokine produced after interactions with bacterial pathogens, may also alter tight junctions by altering the transcription for the proteins mentioned previously (7). In addition to altering transcription of tight junction proteins, TNF-α has been shown to alter intestinal epithelial permeability by inducing apoptosis of the enterocytes and preventing tight junctions from acting on and filling the gaps left in the barrier (7). Alterations of the gene transcription and expression for these tight junction proteins may be significant in future studies for treatments of the intestinal injury seen in NEC and with the use of enteroids it is possible to study this phenomenon ex vivo for a deeper understanding of the diagnosis.
The remainder of the intercellular junctions consists of adherence junctions and desmosomes. The adherence junctions are adjacent to tight junctions and assist in cell recognition and mediating intercellular associations (6). Next to the adherence junction is the desmosome, which helps to create stronger intercellular associations (6). Tight junctions in particular remain a noteworthy area of research for the present and future of intestinal disease in NEC.
The intestinal epithelium
As previously mentioned, the intestinal epithelial cells are made up of four major cell types: the enterocytes, goblet cells, Paneth cells and enteroendocrine cells (6-8). The enterocytes form the single, columnar layer of cells held together by the intercellular junctions and maintain the selectively permeable nature of the barrier (6,8,19). Goblet cells are single cell glands differentiated from intestinal epithelial cells that are responsible for the secretion of mucins to form the mucus layer of the intestinal barrier (6-8,13,19). Goblet cell depletion and alteration of function has been demonstrated in studies looking at the intestine of NEC patients which is implicated in the decreased mucus layer seen in the intestinal injury characteristic of NEC (23).
Paneth cells remain in the intestinal crypts and are responsible for maintaining intestinal stem cells and villi development through secreting growth factors such as epidermal growth factor, transforming growth factor-β, and Wnt3 (19). Paneth cells also secrete anti-microbial agents such as phospholipase A2, lysozymes, and α-defensins and immunomodulators into the mucus layer to aid in prevention of bacterial translocation (19). Paneth cells fully mature around 35–40 weeks of gestation, leaving premature infants without a fully functional intestinal epithelial cell barrier if born before this time (19,27-29). The lack of mature Paneth cells leads to alterations in the interactions of the intestinal barrier with the microbiome and alters the mechanism by which the intestinal epithelial cells repair themselves. Studies have shown a decrease in Paneth cells in infants with NEC compared to controls (19,27-29). However, some other studies have demonstrated an increase in factors such as α-defensin in some infants that developed NEC, which may have demonstrated a difference in surgical resection timing, however this highlights the fact that NEC is a complex diagnosis and the need for continued research is critical (28).
The fourth cellular component of the intestinal epithelium, the enteroendocrine cells, maintains homeostasis by responding to signals from neurons and microbes to secrete regulatory peptide hormones (19). This helps to modulate interactions with the gut microbiome and removal of pathogens. They also release chemokines and defensins similar to Paneth cells (19). Through their maintenance of the epithelial cell barrier enteroendocrine cells may play a role in intestinal injury prevention, however there is a lack of studies comparing these cells in patients with NEC to controls indicating potential future research opportunities (19).
The microbiome
The microbiome consists of diverse microorganisms in the gastrointestinal tract which communicate with the intestinal epithelial cells and immune cells to regulate immune responses and maintain homeostasis (11). Alterations of the microbiome, whether it be low microbial diversity or an overabundance of bacteria, can lead to significant problems for the intestinal epithelial cells (11). Microbial dysbiosis is frequently seen in premature neonates and can be attributed to multiple factors.
Due to the under-development of the aforementioned intestinal epithelial cells, the premature infant intestinal barrier exhibits increased “leakiness” at baseline in contrast to a mature intestinal barrier (1,9,10). The mature intestine has many other factors that act as a defense against pathologic bacteria. The mature intestine has coordinated peristalsis, balanced gastric acid secretion, proteolytic enzymes, and the intestinal barrier itself is more mature exhibiting mature mucus layer, epithelial cells, and tight junctions (14,30,31). Intestinal motility usually develops during the third trimester and the migratory motor complexes typically develop around 34 weeks gestation (9). Premature neonates do not have fully developed gastrointestinal motility and coordinated peristalsis therefore this can lead to bacterial overgrowth due to stasis altering the microbiome (1,9,10,25,30). Studies have shown that the reduction in gut motility was accompanied by an increase in NEC severity as well as an increase in mucosal injury leading to an increased NEC severity score (30).
Having mature functioning gut aids in preventing bacterial stasis and overgrowth leading to pathogen attachment to the intestinal barrier and translocation resulting in an inflammatory response and damage to the epithelial cells and intercellular junctions (14). Adding insult to injury, the premature infants microbiome colonization has been shown to be far less diverse, essentially sterile at birth, compared to the mature microbiome leaving it vulnerable to pathogenic colonization (14). The microbiome of a premature infant is heavily influenced by factors within the neonatal intensive care unit (NICU) such as being in a hospital environment, antibiotic use, acid blockers, and frequent foreign objects entering the gastrointestinal tract such as feeding tubes (14). Having an altered microbiome affects the communication between the native microbial species and the intestinal epithelium, potentially leading to adverse function of this barrier which can trigger an exaggerated inflammatory response (14).
Studies have shown that premature intestine have an upregulated bacterial receptor called toll-like receptor 4 (TLR4) which is a receptor for LPS on gram negative bacteria (10,32). TLR4 is part of the innate immune system and is present on immune cells, intestinal epithelium, and the endothelium of intestinal mesentery, it is involved in regulating cell migration, proliferation, and apoptosis (10,32). When occurring in a regulated fashion, activation of TLR4 initiates an inflammatory response leading to recruitment of immune factors and elimination of the pathogen (10). Having this upregulated in addition to the previously mentioned changes in the premature intestine results in increased bacterial translocation (10). Due to this, TLR4 has become a point of interest for research in the intestinal injury of NEC diagnoses. TLR4 has been shown to have relevance to the pathogenesis of NEC both in human tissue and animal models of NEC (32). In animal models, it has been shown that TLR4 deficient mice are protected from the intestinal injury seen in NEC (32). It is thought that the recognition of substances, such as LPS, by TLR4 leads to eventual intestinal epithelial cell barrier injury, activation of a hyperinflammatory response, induction of apoptosis, and inhibition of mucosal repair resulting in increased permeability and further bacterial translocation (10,30-32). LPS interaction stimulates the translation of pro-inflammatory cytokines, such as TNF-α mentioned previously, and interleukins 6 and 8 (31). This stimulates migration of neutrophils and proinflammatory T helper 17 cell (Th17) lymphocytes leading to further inflammation and damage to the intestinal barrier increasing permeability and allowing continued bacterial translocation (1,10,32). After injury and bacterial translocation, LPS interacts with TLR4 on the endothelium of the intestinal mesentery resulting in a decrease of endothelial nitric oxide (NO) synthase which generates the vasodilatory molecule NO leading to intestinal ischemic insult (10,30-32). This disruption in the vasculature of the intestine decreases the ability of the intestinal epithelium to repair itself, allowing further bacterial translocation and perpetuating the cycle of intestinal injury (Figure 2) (10,30,31).
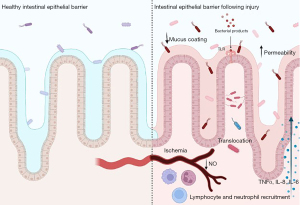
Interactions with LPS have also been shown to physically alter components of the intercellular junctions between epithelial cells therefore altering permeability (12,33). The intercellular junctions change rapidly in response to inflammation, this can be beneficial when occurring in a regulated fashion, however in a hyperinflammatory state this can be detrimental to the permeability of the barrier (12,33). Tight junctions in particular have been shown to undergo alterations in intestinal injury through interactions with LPS (12,33). As mentioned previously, LPS has been shown to change expression and arrangement of many tight junction proteins, including claudins, ZO-1, and by downregulating expression of the proteins (1,9,33). Microbial dysbiosis seen in premature neonates may result an altered inflammatory balance leading to an increase in potential pathogenic bacteria and bacterial ligands, such as LPS, leading to alterations in the intestinal membrane through mechanisms described above (12).
Possible preventative measures
While there is no cure for NEC, there have been several approaches for prevention of the intestinal injury seen in NEC over time. One of the most notable components in the effort for prevention of NEC has been the administration of breast milk (1-3,10,25). Many studies have sought to understand the molecular components of breast milk that offer protection against intestinal injury. The antimicrobial products of breast milk such as immunoglobulins, cytokines, lactoferrin, and oligosaccharides all have been implicated in having a positive impact on prevention of NEC (1-3,10,25). In addition to those, there is a high level of prebiotics that are present in colostrum that may mitigate bacterial translocation and factors that likely facilitate the maturation of the immune system (1-3,10,25). Investigators have compared breast milk feeding in premature infants versus formula fed premature infants and found that the breast milk fed infants have a significant reduction in the incidence of NEC intestinal injury (1-3,10,25). It is thought that breast milk prevents NEC through decreasing intestinal permeability, enhancing the immature physical barrier, and aids in natural bacterial colonization preventing overgrowth by potential pathogens (1,34).
Probiotics have also been studied as potential trial therapies for prevention of NEC. Probiotics may help in prevention of intestinal injury through maintaining balance of the microbiome which would aid in the overall gut health resulting in less bacterial translocation through the “leaky” premature intestine (9). However, further studies are needed regarding probiotics and intestinal injury prevention.
Treatment strategies
Although medical management with bowel rest, intravenous antibiotics and, if needed, surgery, remain the mainstay in the treatment of patients with NEC intestinal injury, future approaches may include strategies to improve intestinal barrier function. Future potential therapies utilizing breast milk and amniotic fluid stem cells are being explored to counteract inflammation and increased permeability leading to NEC intestinal injury (2). It has been shown that there is a family of TLR4 inhibitors present in breast milk, which could partially explain the preventative and therapeutic qualities of breast milk in NEC (2,10). Treatment strategies exploring TLR4 inhibition similar to that in breast milk are being studied and have potential for future therapeutic medications to ameliorate the inflammatory pathway leading to increased permeability. Further research is essential to develop treatment strategies and studying the intestinal barrier may be of great importance in discovering future prevention and treatment plans.
Strengths and limitations
This paper provides an up to date review on the current understanding of the intestinal barrier in the pathogenesis of NEC. However, a limitation of this review is that NEC is a multifactorial process and there may be overlap due to other etiologies. Some of the studies referenced are cell or animal based and may not be truly reflective in the disease process in humans which highlights the need for further research in the area.
Conclusions
The intestinal barrier is complex and of utmost importance in the pathogenesis of intestinal injury seen in NEC. The overlying mucus layer and the epithelial cell barrier components, including the varying cell types and intercellular junctions, all contribute to the permeability of the intestinal barrier. These various components of the intestinal barrier are more vulnerable during prematurity, which is when the bulk of NEC diagnoses are seen. There have been large strides made in understanding the interactions of bacterial pathogens and receptors leading to alterations in the barrier, however there is much to be discovered in regards to the diagnosis of NEC. Continuing to grow in our understanding of the intestinal barrier, intestinal epithelium, and the microbiome will aid in developing prevention and treatment strategies of the intestinal injury seen in NEC.
Acknowledgments
Funding: This study was supported by Oklahoma Center for Adult Stem Cell Research (Dr. Catherine J. Hunter) (OCASCR Grant Number 231013).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Josef Neu) for the series “Necrotizing Enterocolitis” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-22-41/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-22-41/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-22-39/coif). The series “Necrotizing Enterocolitis” was commissioned by the editorial office without any funding or sponsorship. C.J.H. was funded by the Oklahoma Center for Adult Stem Cell Research (Dr. Catherine J. Hunter); however, she does not have conflicts of interest with this funding. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hunter CJ, Upperman JS, Ford HR, et al. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 2008;63:117-23. [Crossref] [PubMed]
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255-64. [Crossref] [PubMed]
- Alganabi M, Lee C, Bindi E, et al. Recent advances in understanding necrotizing enterocolitis. F1000Res 2019;8:F1000 Faculty Rev-107.
- Ravisankar S, Tatum R, Garg PM, et al. Necrotizing enterocolitis leads to disruption of tight junctions and increase in gut permeability in a mouse model. BMC Pediatr 2018;18:372. [Crossref] [PubMed]
- Pearlman SA. Advancements in neonatology through quality improvement. J Perinatol 2022;42:1277-82. [Crossref] [PubMed]
- Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol 2008;14:401-7. [Crossref] [PubMed]
- Michielan A, D'Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm 2015;2015:628157. [Crossref] [PubMed]
- Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 2005;306:357-63. [Crossref] [PubMed]
- Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med 2011;16:145-50. [Crossref] [PubMed]
- Hackam DJ, Sodhi CP. Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis. Cell Mol Gastroenterol Hepatol 2018;6:229-238.e1. [Crossref] [PubMed]
- Lemme-Dumit JM, Song Y, Lwin HW, et al. Altered Gut Microbiome and Fecal Immune Phenotype in Early Preterm Infants With Leaky Gut. Front Immunol 2022;13:815046. [Crossref] [PubMed]
- Stephens M, von der Weid PY. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020;11:421-32. [Crossref] [PubMed]
- Zhang M, Wu C. The relationship between intestinal goblet cells and the immune response. Biosci Rep 2020;40:BSR20201471. [Crossref] [PubMed]
- Claud EC. Neonatal Necrotizing Enterocolitis -Inflammation and Intestinal Immaturity. Antiinflamm Antiallergy Agents Med Chem 2009;8:248-59. [Crossref] [PubMed]
- Lin J, Holzman IR, Jiang P, et al. Expression of intestinal trefoil factor in developing rat intestine. Biol Neonate 1999;76:92-7. [Crossref] [PubMed]
- Udall JN Jr. Gastrointestinal host defense and necrotizing enterocolitis. J Pediatr 1990;117:S33-43. [Crossref] [PubMed]
- van Elburg RM, Fetter WP, Bunkers CM, et al. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed 2003;88:F52-5. [Crossref] [PubMed]
- Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol 2017;11:821-34. [Crossref] [PubMed]
- Demers-Mathieu V. The immature intestinal epithelial cells in preterm infants play a role in the necrotizing enterocolitis pathogenesis: A review. Health Sciences Review 2022;4:100033. [Crossref]
- Liu D, Xu Y, Feng J, et al. Mucins and Tight Junctions are Severely Altered in Necrotizing Enterocolitis Neonates. Am J Perinatol 2021;38:1174-80. [Crossref] [PubMed]
- Beach RC, Menzies IS, Clayden GS, et al. Gastrointestinal permeability changes in the preterm neonate. Arch Dis Child 1982;57:141-5. [Crossref] [PubMed]
- Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr 2007;85:629S-34S. [Crossref] [PubMed]
- Vieten D, Corfield A, Carroll D, et al. Impaired mucosal regeneration in neonatal necrotising enterocolitis. Pediatr Surg Int 2005;21:153-60. [Crossref] [PubMed]
- Moore SA, Nighot P, Reyes C, et al. Intestinal barrier dysfunction in human necrotizing enterocolitis. J Pediatr Surg 2016;51:1907-13. [Crossref] [PubMed]
- Duchon J, Barbian ME, Denning PW. Necrotizing Enterocolitis. Clin Perinatol 2021;48:229-50. [Crossref] [PubMed]
- Bein A, Eventov-Friedman S, Arbell D, et al. Intestinal tight junctions are severely altered in NEC preterm neonates. Pediatr Neonatol 2018;59:464-73. [Crossref] [PubMed]
- Coutinho HB, da Mota HC, Coutinho VB, et al. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J Clin Pathol 1998;51:512-4. [Crossref] [PubMed]
- Lueschow SR, McElroy SJ. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front Immunol 2020;11:587. [Crossref] [PubMed]
- McElroy SJ, Prince LS, Weitkamp JH, et al. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2011;301:G656-66. [Crossref] [PubMed]
- Kovler ML, Gonzalez Salazar AJ, Fulton WB, et al. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci Transl Med 2021;13:eabg3459. [Crossref] [PubMed]
- Nanthakumar NN, Fusunyan RD, Sanderson I, et al. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A 2000;97:6043-8. [Crossref] [PubMed]
- Hackam DJ, Sodhi CP. Bench to bedside - new insights into the pathogenesis of necrotizing enterocolitis. Nat Rev Gastroenterol Hepatol 2022;19:468-79. [Crossref] [PubMed]
- Bein A, Zilbershtein A, Golosovsky M, et al. LPS Induces Hyper-Permeability of Intestinal Epithelial Cells. J Cell Physiol 2017;232:381-90. [Crossref] [PubMed]
- Gunasekaran A, Eckert J, Burge K, et al. Hyaluronan 35 kDa enhances epithelial barrier function and protects against the development of murine necrotizing enterocolitis. Pediatr Res 2020;87:1177-84. [Crossref] [PubMed]
Cite this article as: Snyder KB, Hunter CJ. The leaky gut: a narrative review on the role of epithelial cell permeability in necrotizing enterocolitis. Pediatr Med 2024;7:20.

