
Reference ranges for SpO2, respiratory rate, and tidal volume in term newborn infants after birth: a narrative review
Introduction
During fetal life, the mean arterial partial pressure of oxygen (paO2) ranges from 25 to 40 mmHg. The umbilical vein containing oxygenated blood is re-directed through specific intra-and-extracardiac shunts (in the foramen ovale and ductus arteriosus) to the brain and myocardium. The first and most critical step in establishing airborne respiration after birth requires the clearance of the liquid filling the lungs. The first profound breaths generate a negative transthoracic pressure relative to the atmospheric pressure (ΔPAa: –40 and –50 cmH2O). The transthoracic pressure constitutes the driving force that extrudes the fluid filling the lungs to the interstitial space and contributes to the aeration of the alveolar space, thus facilitating the alveolar-capillary gas exchange. Additionally, braking maneuvers, crying, and the surfactant lining the alveolar surface contribute to the establishment of a functional residual capacity (FRC).
The increment of the arterial blood oxygen content is a major factor that causes lung vasculature dilatation, a drop in pulmonary vascular resistance and increased pulmonary blood flow. Consequently, the entire right ventricular output is re-directed through the lungs and replaces the right umbilical venous return as the main source of the left ventricular preload after the umbilical cord is either clamped or flow spontaneously ceases (1-5).
This review sought to describe the physiological steps that occur in the first minutes after birth that contribute to air respiration and adult-type circulation. We also describe the recently updated reference ranges for respiratory parameters, heart rate (HR), and arterial oxygen saturation (SpO2). Reference ranges are a valuable guide for neonatologists to provide optimal ventilation and oxygenation to newborn infants during the fetal-to-neonatal transition when resuscitation maneuvers are requested. We present this article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-75/rc).
Methods
Data on the respiratory function and oxygenation pattern of normal term infants in the stabilization period during the first minutes after birth were retrieved and stored by employing continuous pre-ductal pulse oximetry and respiratory function monitoring. The electronic databases searched included MEDLINE, Embase, CINAHL, and Scopus (see Table 1). Statistical methods were employed to establish the percentile curves for each parameter.
Table 1
| Items | Specification |
|---|---|
| Date of search | 30/6/2022 |
| Databases and other sources searched | MEDLINE, Embase, CINAHL, and Scopus |
| Search terms used | Oxygen saturation, HR, RR, VT, term infants, delivery, reference ranges, and post-natal adaptation |
| Timeframe | 01/01/2010–30/6/2022 |
| Inclusion criteria | Prospective observational studies that included monitoring of physiological parameters in the delivery room (heart rate, oxygen saturation, respiratory rate, tidal volume) |
| Retrieval and analysis of data | |
| Articles in English-language | |
| Selection process | Independent selection by 2 authors with a data extraction form |
VT, tidal volume; HR, heart rate; RR, respiratory rate.
Lung-based respiration after birth
Initiation of respiration
During intrauterine life, fetal breathing activity is intermittently present and largely contributes to lung growth and development (6). The mechanisms that regulate the change from fetal intermittent respiration to newborn continuous respiration are yet unknown. However, a series of factors have been identified that notably affect the initiation of a regular respiratory pattern after birth. The role of chemoreceptors responding to hypercarbia and/or hypoxia in the first minutes after birth and especially in pre-term infants is yet unclear. Thus, while hypoxia inhibits respiratory efforts in utero, it appears to be a potent stimulator in new born infants (7,8). Conversely, pre-term infants display an immature response to hypoxia that appears to suppress respiratory activity (7,8).
Oxygen supplementation, especially the use of pure oxygen, has a negative effect on the respiratory drive, especially after hypoxic-ischemic events, such as in asphyxia. The initiation of diaphragmatic contractions and breathing activity occurred earlier in asphyctic rats ventilated with a lower than those ventilated with a higher inspired fraction of oxygen (9). Additionally, in a series of studies performed in asphyxiated term newborn infants, it was shown that the time to the first cry and/or initiation of spontaneous respiration was achieved earlier when newborn infants were ventilated with room air instead of 100% oxygen (10-12).
As van Vonderen et al. (5) noted: “Physical stimuli are also relevant to trigger respiration. Hence, to lower the environmental temperature, rubbing the newborn’s back, or using bright lights, may trigger respiratory efforts that may contribute to ensuring a regular pattern of respiration”.
Aerating the lung, and creating an FRC
In utero, the continuous secretion of fluid contributes to keeping the lung distended, which further promotes lung growth and development. Conversely, a reduction in lung fluid production leads to lung collapse and the arrest of lung growth (7). Airborne breathing requires the clearing of the lungs in a few minutes to allow alveolar-capillary gas exchange. Currently, the first inspiratory movements are largely considered the exclusive mechanism for lung fluid extrusion. The first intense respiratory efforts generate a transpulmonary pressure that forces the fluid filling the airways across the alveoli, especially type I pneumocytes, to the interstitium (13,14). Additionally, stress induced by fetal expulsion favors the release of adrenaline that stimulates the epithelial cells to activate luminal surface sodium channels. This reverses the Na+ flux and the osmotic gradient across the epithelium, causing the reabsorption of the lung liquid. The relevance of sodium channels has been challenged by studies using phase X-ray imaging that clearly show liquid clearance and lung aeration during the first inspiratory movements (15). Finally, the role of aquaporins does not appear to be related to the rapid clearance of fluid immediately after birth but with slow ongoing clearance in the following days (16). During expiration, air retention in the lungs is caused by the end expiratory pressure secondary to the closure of the glottis, breath holds, and/or crying (4). Additionally, surfactant rapidly expands on the alveolar surface and drastically reduces the tensioactive forces, thus preventing lung collapse during expiration and increasing the uniformity of lung aeration contributing to the formation of a FRC (17,18). FRC is essential to establish effective airborne respiration.
Reference range for respiratory parameters after birth
Approximately 5–10% of all newborn infants will require respiratory assistance in the first minutes after birth. Lung ventilation is the cornerstone of neonatal resuscitation (19). Positive pressure ventilation (PPV) adequately performed homogeneously expands the lungs, facilitates lung fluid extrusion to the interstitial space, creates a FRC and normalizes blood gases (20). However, the optimization of ventilation and avoidance of lung damage requires that the respiratory parameters be kept within the physiologic ranges. For this purpose, the monitoring of the tidal volume (VT) is more effective during PPV than the monitoring of the peak inspiratory pressure (PIP) (21,22). Thus, Kattwinkel et al. (22), proved that monitoring VT was superior to monitoring pressure to detect changes of lung compliance during mechanical ventilation.
Additionally, excessive VT even for short periods was shown to cause lung overdistension and damage in experimental studies (23). For example, administering 6 manual inflations of 35–40 mL/kg to lambs, which represents a VT 6 times greater than average (6 mL/kg), hindered their response to rescue surfactant and caused important changes in lung cytoarchitecture (23). In a rabbit model, Hernandez et al. (24) showed that overdistension caused by high VT rather than PIP was responsible for lung damage. Additionally, it has been shown that a wide range of VT can be delivered by employing a fixed PIP (21). Consequently, adjusting VT according to lung compliance within a safe volume range allows the desired PIP to be reached while minimizing lung overdistension.
Recently, Baixauli-Alacreu et al. (25) provided a reference range for the VT and respiratory rate (RR). In a prospective observational study, these authors employed a respiratory function monitor (RFM) to retrieve the VT and RR in healthy spontaneously breathing term newborn babies who did not require resuscitation maneuvers and had been born by C-section during post-natal stabilization in the delivery room (DR). The RFM sensors for respiratory parameters and flow were connected to the mask interface, and the mask was sealed to ensure optimal hold during the monitoring. The mask was open to air with neglectable expiratory resistance (25). A total of 243 term newborn infants were included in the study. A total of 59,058 valid observations representing 32,801 breaths were subjected to breath-by-breath analysis using an ad-hoc software for respiratory research (Pulmochart; Advance Life Diagnostics, Germany). The percentiles for the VT and RR were determined and graphically represented (see Figures 1,2, respectively). The median and interquartile ranges (IQRs) for the VT at 2, 5, and 10 min were 4.2 [1.9–6.5], 4.9 [1.9–7.3], and 4.6 [1.9–6.6] mL/kg, respectively, and those for the RR were 69 [53–82], 76 [60–90], and 78 [61–92] breaths per minute (bpm), respectively.
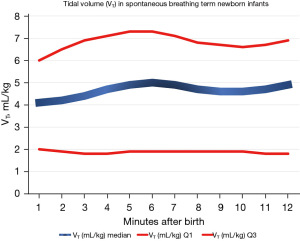
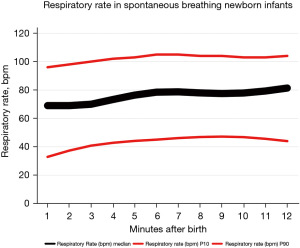
Some interesting conclusions were drawn from this study. Notably, females had a significantly greater VT at the 3 time points (2, 5, and 10 min) after birth, which may indicate, as has previously been suggested, that pulmonary maturation is more delayed in males than females (25,26). Additionally, a significant correlation between the evolving VT and RR (P=0.96), and the VT and SpO2 (P=0.83) was established. We envision that in the future, optimal ventilation will be guided by airway pressure, gas flow, mask leak, and, most importantly, by the VT.
Oxygenation after birth
Both the initiation of lung respiration and cord clamping have critical effects on oxygenation. Lung respiration and alveolar-capillary gas exchange enhance blood oxygenation flowing into the left atrium. Delayed cord clamping (DCC) increases blood hemoglobin content and right ventricular preload. Both circumstances will increase the availability of oxygen to tissue (2).
Pulse oximetry monitoring during post-natal adaptation
The use of pulse oximeters (POs) notably facilitates the monitoring of SpO2. POs are easy to manage and do not require tedious calibration procedures. The use of POs became generalized in the DR in the 1980s in infants to assess SpO2 during post-natal stabilization (27). A body of evidence has been rapidly gathered. The SpO2 of 1,732 term newborn infants enrolled in 10 observational studies was recorded between 1987 and 2010 (28). In these studies, SpO2 was continuously recorded during the first minutes after birth, and it was noted that in the first 2–3 min after birth, the SpO2 was around 75%, but increased rapidly, achieving stable values >90% at 7–8 min after birth (28). Additionally, it was also stated that the post-ductal SpO2 was 2–3% lower than the pre-ductal SpO2, and this difference could persist for at least 24–48 hours after birth (29). Additionally, it was also established that babies born by C-section exhibited lower SpO2 in the first minutes after birth than those born by vaginal delivery (30).
Reference range for oxygen saturation and HR after birth
In 2010, Dawson et al. (31), established a reference range by merging 3 pre-ductal SpO2 data sets from the Royal Women’s Hospital (Melbourne) and the Hospital Universitario y Politécnico La Fe (Valencia) that included 468 babies (ranging from 25 to 40 weeks of gestation) whose SpO2 and HR had been continuously monitored for the first 10 min after birth. The newborn infants included in the study had not been resuscitated after birth. The SpO2 and HR data were recorded every 2 seconds with maximum sensitivity totaling 61,650 SpO2 data points. The population comprised a total of 465 babies, 64% of whom were term infants (≥37 weeks) and 36% of whom were pre-term (<37 weeks). The 3rd, 10th, 50th, 90th, and 97th percentiles minute by minute for the first 10 min after birth were calculated and graphically represented in 3 different graphs for the term (≥37 weeks), late pre-term (32–36 weeks), and very pre-term (<32 weeks) infants (31). Dawson’s reference ranges have been widely employed since their publication in 2010 (31). In 2015, the American Heart Association (AHA) recommended the following target ranges for SpO2: at 1 min 60–65%, at 2 min 65–70%, at 3 min 70–75%, at 4 min 75–80%, at 5 min 80–85%, and at 10 min 85–95% (32).
Reference ranges for SpO2 and HR in newly born infants with DCC
The 2020 AHA guidelines recommend that “after an uncomplicated term or late pre-term birth, it is reasonable to delay cord clamping until after the baby is placed on the mother, dried, and assessed for breathing, tone, and activity.” (33). In 2018, DCC had already become a standard practice in the DR in Spain as recommended by the Spanish Society for Obstetrics and Gynecology (34). Kc et al. (35) conducted a single-center study in which 1,510 women with uneventful deliveries were randomly assigned to cord clamping in ≤60 s of birth and cord clamping in ≥180 s. The babies with DCC exhibited significantly higher oxygen saturations during the first 10 min after birth; however, the babies with DCC had lower HRs in the first 5–6 min (35).
In 2018, we launched a prospective observational study to establish a normality range for SpO2 and HR using pre-ductal POs in term babies born after an uneventful pregnancy by vaginal delivery and with >60-second DCC (36). We also aimed to compare the results to the Dawson reference range published in 2010 when immediate cord clamping was routine (31). Of the 392 eligible term newborn infants (≥37 weeks’ gestation), we assessed 282 babies and registered a total of 70,257 SpO2 and 79,746 HR measurements. The study was performed using a low noise cabled pulse oximetry sensor (Masimo SET Masimo-LNCS; Irvine; CA), which was placed on the infant’s right hand or wrist for pre-ductal SpO2 and HR. Immediately after placing the sensor, the cable was connected to the oximeter (Pulsi CO-Oximeter Radical 7 touch screen Masimo, Irvine, CA). The SpO2 and HR were set at 2-second intervals and maximal sensitivity. Once the PO had been connected, the baby was put in the prone position on the mother’s chest and covered to avoid hypothermia for 45 min. The cord was left patent for a median of 110±62 seconds. The results for the 3rd, 10th, 25th, 50th, 75th, 90th, and 97th percentiles for SpO2 and HR are shown in Figure 3, and the minute-by-minute data are shown in Tables 2,3 (31). DCC significantly modified the reference ranges for SpO2, especially in the first 5 min after birth. As Table 2 shows, the reference range [median (IQR)] for SpO2 was significantly higher (P<0.001) during the first 5 min after birth in neonates with DCC, but no differences were observed compared to the Dawson reference range from the 5th minute onwards. The HR of the neonates with DCC showed more stable values (which remained between 148–157 bpm), especially in the first 2–3 min, while the neonates with immediate cord clamping evolved from 99 bpm at 1 min to 160 at the 3rd min after birth as shown in Dawson’s nomogram (see Table 3).
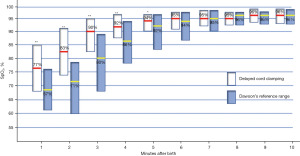
Table 2
| Minutes after birth | DCC group (n=282), median [IQR] | Dawson’s reference range (n=246), median [IQR] | P value |
|---|---|---|---|
| 1 | 77 [68–85] | 67 [62–76] | <0.001 |
| 2 | 83 [74–91] | 71 [60–78] | <0.001 |
| 3 | 90 [82–95] | 80 [68–89] | <0.001 |
| 4 | 92 [88–96] | 86 [78–94] | <0.001 |
| 5 | 94 [90–96] | 164 [147–180] | 0.04 |
| 6 | 95 [91–97] | 94 [87–97] | 0.19 |
| 7 | 95 [92–97] | 95 [90–97] | 0.21 |
| 8 | 95 [92–98] | 96 [92–98] | 0.45 |
| 9 | 96 [94–98] | 96 [93–97] | 0.30 |
| 10 | 96 [93–98] | 96 [93–98] | 0.23 |
SpO2, arterial oxygen saturation; IQR, interquartile range; DCC, delayed cord clamping.
Table 3
| Minutes after birth | DCC group (n=282), median [IQR] | Dawson’s reference range (n=246), median [IQR] | P value |
|---|---|---|---|
| 1 | 148 [84–170] | 99 [66–132] | <0.001 |
| 2 | 154 [124–169] | 144 [115–171] | <0.001 |
| 3 | 157 [143–170] | 160 [138–180] | <0.001 |
| 4 | 158 [144–170] | 163 [145–181] | <0.001 |
| 5 | 155 [143.167] | 164 [147–180] | <0.001 |
| 6 | 152 [141–168] | 163 [147–179] | <0.001 |
| 7 | 153 [142–163] | 162 [146–178] | <0.001 |
| 8 | 152 [140–164] | 159 [144–173] | <0.001 |
| 9 | 152 [142–161] | 157 [143–172] | <0.001 |
| 10 | 151 [141–161] | 157 [142–171] | <0.001 |
HR, heart rate; IQR, interquartile range; DCC, delayed cord clamping.
Final considerations and conclusions
In recent years, researchers in the field of fetal-to-neonatal transition and newborn resuscitation have been seeking to incorporate objectivity into their interventions in the DR. Thus, it is essential that reference ranges for the most vital clinical signs are established. Baixauli-Alacreu et al. (25) were the first to construct a reference range for the VT and RR for term newborn infants. This study should be further developed and include late pre-term and extreme preterm infants to adequately guide ventilation during post-natal stabilization. The 2010 reference range for HR and SpO2 elaborated by Dawson and his coworkers set the basis for guiding the oxygenation of newborn infants (31). The reference range developed by Padilla-Sánchez et al. (36), showed that DCC for approximately 2 min generates substantial changes in SpO2 and HR, especially in the first 5 min after birth, which should be taken into consideration in the new resuscitation guidelines.
In the future, caregivers in the DR will likely have access to precise and valuable information in real time that will objectively guide their interventions and thus reduce iatrogenic damage and increase effectiveness.
Acknowledgments
Funding: This work was supported by grants from the Health Research Institute Carlos III (Ministry of Science and Innovation; Kingdom of Spain) (Nos. PI20/00964, RD16/0022/0001, and ILC CM20/00187 to M.V.).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ola Didrik Saugstad) for the series “Oxygen in the Newborn” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-75/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-75/coif). The series “Oxygen in the Newborn” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vento M, Teramo K. Evaluating the fetus at risk for cardiopulmonary compromise. Semin Fetal Neonatal Med 2013;18:324-9. [Crossref] [PubMed]
- Vali P, Lakshminrusimha S. The Fetus Can Teach Us: Oxygen and the Pulmonary Vasculature. Children (Basel) 2017.
- Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev 2010;90:1291-335. [Crossref] [PubMed]
- Lara-Cantón I, Solaz A, Parra-Llorca A, et al. Optimal Inspired Fraction of Oxygen in the Delivery Room for Preterm Infants. Children (Basel) 2019.
- van Vonderen JJ, Roest AA, Siew ML, et al. Measuring physiological changes during the transition to life after birth. Neonatology 2014;105:230-42. [Crossref] [PubMed]
- Hooper SB, Harding R. Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung. Clin Exp Pharmacol Physiol 1995;22:235-47. [Crossref] [PubMed]
- Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. J Appl Physiol (1985) 1996;81:209-24. [Crossref] [PubMed]
- Davey MG, Moss TJ, McCrabb GJ, et al. Prematurity alters hypoxic and hypercapnic ventilatory responses in developing lambs. Respir Physiol 1996;105:57-67. [Crossref] [PubMed]
- Bookatz GB, Mayer CA, Wilson CG, et al. Effect of supplemental oxygen on reinitiation of breathing after neonatal resuscitation in rat pups. Pediatr Res 2007;61:698-702. [Crossref] [PubMed]
- Saugstad OD, Rootwelt T, Aalen O. Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics 1998;102:e1. [Crossref] [PubMed]
- Vento M, Asensi M, Sastre J, et al. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 2001;107:642-7. [Crossref] [PubMed]
- Vento M, Asensi M, Sastre J, et al. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr 2003;142:240-6. [Crossref] [PubMed]
- Siew ML, Wallace MJ, Kitchen MJ, et al. Inspiration regulates the rate and temporal pattern of lung liquid clearance and lung aeration at birth. J Appl Physiol (1985) 2009;106:1888-95. [Crossref] [PubMed]
- Hooper SB, Roberts C, Dekker J, et al. Issues in cardiopulmonary transition at birth. Semin Fetal Neonatal Med 2019;24:101033. [Crossref] [PubMed]
- Hooper SB, Te Pas AB, Kitchen MJ. Respiratory transition in the newborn: a three-phase process. Arch Dis Child Fetal Neonatal Ed 2016;101:F266-71. [Crossref] [PubMed]
- Escobar J, Gormaz M, Arduini A, et al. Expression of aquaporins early in human pregnancy. Early Hum Dev 2012;88:589-94. [Crossref] [PubMed]
- Whitsett JA. The molecular era of surfactant biology. Neonatology 2014;105:337-43. [Crossref] [PubMed]
- Siew ML, Te Pas AB, Wallace MJ, et al. Surfactant increases the uniformity of lung aeration at birth in ventilated preterm rabbits. Pediatr Res 2011;70:50-5. [Crossref] [PubMed]
- Schmölzer GM, Kamlin OC, Dawson JA, et al. Respiratory monitoring of neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed 2010;95:F295-303. [Crossref] [PubMed]
- Schmölzer GM, Morley CJ, Wong C, et al. Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J Pediatr 2012;160:377-381.e2. [Crossref] [PubMed]
- Schmölzer GM, Te Pas AB, Davis PG, et al. Reducing lung injury during neonatal resuscitation of preterm infants. J Pediatr 2008;153:741-5. [Crossref] [PubMed]
- Kattwinkel J, Stewart C, Walsh B, et al. Responding to compliance changes in a lung model during manual ventilation: perhaps volume, rather than pressure, should be displayed. Pediatrics 2009;123:e465-70. [Crossref] [PubMed]
- Björklund LJ, Ingimarsson J, Curstedt T, et al. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res 1997;42:348-55. [Crossref] [PubMed]
- Hernandez LA, Peevy KJ, Moise AA, et al. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J Appl Physiol (1985) 1989;66:2364-8. [Crossref] [PubMed]
- Baixauli-Alacreu S, Padilla-Sánchez C, Hervás-Marin D, et al. Expired tidal volume and respiratory rate during postnatal stabilization of newborn infants born at term via cesarean delivery. J Pediatr:X 2021;6:100063. [Crossref] [PubMed]
- Seaborn T, Simard M, Provost PR, et al. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab 2010;21:729-38. [Crossref] [PubMed]
- Finer N, Leone T. Oxygen saturation monitoring for the preterm infant: the evidence basis for current practice. Pediatr Res 2009;65:375-80. [Crossref] [PubMed]
- Gottimukkala SB, Sotiropoulos JX, Lorente-Pozo S, et al. Oxygen saturation (SpO2) targeting for newborn infants at delivery: Are we reaching for an impossible unknown?. Semin Fetal Neonatal Med 2021;26:101220. [Crossref] [PubMed]
- Mariani G, Dik PB, Ezquer A, et al. Pre-ductal and post-ductal O2 saturation in healthy term neonates after birth. J Pediatr 2007;150:418-21. [Crossref] [PubMed]
- Kamlin CO, O'Donnell CP, Davis PG, et al. Oxygen saturation in healthy infants immediately after birth. J Pediatr 2006;148:585-9. [Crossref] [PubMed]
- Dawson JA, Kamlin CO, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010;125:e1340-7. [Crossref] [PubMed]
- Wyckoff MH, Aziz K, Escobedo MB, et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S543-60. [Crossref] [PubMed]
- Aziz K, Lee CHC, Escobedo MB, et al. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2021;147:e2020038505E. [Crossref] [PubMed]
- Sociedad Española de Ginecología y Obstetricia. Prenatal control of normal pregnancy. Progresos Obstet y Ginecol 2018;167:687-8.
- Kc A, Singhal N, Gautam J, et al. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth - randomized clinical trial. Matern Health Neonatol Perinatol 2019;5:7. [Crossref] [PubMed]
- Padilla-Sánchez C, Baixauli-Alacreu S, Cañada-Martínez AJ, et al. Delayed vs Immediate Cord Clamping Changes Oxygen Saturation and Heart Rate Patterns in the First Minutes after Birth. J Pediatr 2020;227:149-156.e1. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)
Cite this article as: Padilla-Sánchez C, Baixauli-Alacreu S, Solaz-García Á, Lara-Cantón I, Parra-Llorca A, Vento M. Reference ranges for SpO2, respiratory rate, and tidal volume in term newborn infants after birth: a narrative review. Pediatr Med 2024;7:6.

