
Anti-U hemolytic disease of the fetus and newborn managed by multiple intrauterine transfusions: a case report and review of the literature
Introduction
The U-antigen was first identified by Wiener et al. in 1953 following a fatal hemolytic transfusion reaction (1). In this and a subsequent report they observed near universal expression of the U antigen excepting ~1.2% U-negative subjects of African descent (1-4). Weiner et al. also identified an association between the MNS (formerly MN) blood group and the U-antigen, a finding further substantiated by Greenwalt and colleagues report that U-negative individuals did not react to anti-S or anti-s antibodies (2,3). As reviewed by Reid, the MNSs antigens comprise the second most complex blood antigen group following the Rh blood group (5). These antigens are located on two sialoproteins known as glycophorin A (GPA) and glycophorin B (GPB). The U-antigen is specific to GPB. The function of these proteins is to provide the glycocalyx of the red blood cell (RBC) an overall net negative charge to prevent adhesion to other RBCs, endothelium, and some organisms (5). As reviewed by Anstee, the absence or variance of GPA and GPB have been shown to confer resistance against malarial infection (6). The spectrum of IgG-mediated anti-U hemolysis includes alloimmune disease of the fetus and newborn. The clinical presentation among affected newborns ranges from mild anemia to erythroblastosis fetalis (7).
We present a case of severe fetal anemia requiring multiple intrauterine transfusions (IUTs) in the setting of maternal anti-U positivity. Uniquely, this case reports the lowest critical maternal anti-U titer to-date resulting in clinically significant fetal anemia. We also provide a review of available cases on anti-U hemolytic disease of the fetus and newborn. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/pm-21-72) (8).
Case presentation
Our patient’s mother was a 30-year-old female, Gravida 5 Para 2, A+, anti-U positive (1:8), with chronic iron deficiency anemia and obesity (BMI 41). Her obstetric history was: G1—term birth by spontaneous vaginal delivery, G2—spontaneous abortion, G3—term cesarean section for arrest of dilation, and G4—twin fetal demise in context of acute intraamniotic infection at 20+ weeks gestation, chronic placental abruption, anhydramnios, and anti-U antibody (1:16) identified during workup. A complete erythrocyte antigen profile obtained during her fourth pregnancy when she was found to be anti-U positive showed: blood type A, Rh (D+, c+, C−, E−, c+), FY (a−, b−), JK (a+, b−), KEL (k+), LE (a−, b+), and MNS (M−, N+, U−, s−). Antibody titers during the reported pregnancy were 8 at 8 weeks gestation, 32 at 251/7 weeks gestation and 64 at 264/7 weeks gestation. Additional antibody testing performed at four different time points during the prenatal period screened against antigens: B, C, c, E, e, F, Cw, V, K, k, Kpa, Kpb, JSa, JSb, Fya, Fyb, JKa, JKb, Xga, LEa, LEb, S, s, M, N, P1, LUa, and LUb antibodies. Only anti-U was positive at each screen. In the currently reported pregnancy, prenatal screening labs indicated: rubella immunity and no hepatitis B virus, hepatitis C virus, gonorrhea, chlamydia, syphilis, or human immunodeficiency virus (HIV) infection.
She was found to be pregnant with our patient at approximately 8 weeks gestation. The first ultrasound performed at 24+ weeks showed appropriate fetal growth at the 83rd percentile. At 251/7 weeks, she had a critical anti-U titer of 32 and weekly fetal middle cerebral artery (MCA) Doppler were initiated to screen for fetal anemia. At 262/7 weeks, MCA Doppler indices were at the severe range at 50.67 cm/sec [1.49 multiples of the median (MoM)] without fetal hydrops. A repeat MCA Doppler was performed at 265/7 with a peak systolic velocity of 59.91 cm/sec (1.72 MoM). We defined fetal anemia based on values defined by Nicolaides et al. and plotted in Figure 1 (9). Percutaneous umbilical cord blood sampling (PUBS) was performed to assess the degree of anemia and IUT with fresh blood [fresh, cross-matched, leukocyte depleted, cytomegalovirus (CMV) negative, irradiated, high hematocrit (Hct)] was planned if severe anemia was confirmed.
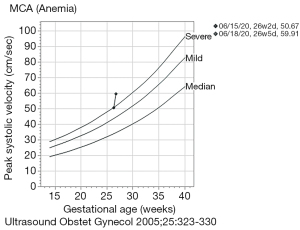
- PUBS performed at 265/7 weeks demonstrated a pre-transfusion peripheral blood count with a fetal Hgb of 8.9 g/dL and a reticulocytosis of 6.7%, documenting severe fetal anemia and an intraoperative point-of-care (HemoCue) hemoglobin (Hgb) of 11.1 (range, 10.9–11.3) g/dL. Based on the data from Nicolaides et al. the <2 SD cutoff for 26 weeks is 10.8 g/dL and for 27 weeks is 11.0 g/dL (9). With such close proximity to these cutoffs and a highly abnormal MCA Doppler we opted to pursue transfusion. Therefore, 40 mL of packed red cells were transfused intravascularly (donor Hgb 19.5 g/dL) with the expectation to raise fetal Hgb to 15 g/dL. However, a post-transfusion Hgb was not obtainable.
- At 283/7 weeks, due to difficulty obtaining fetal intravascular access, a pre-transfusion fetal Hgb was not obtained and transfusion of 80 mL of packed red cells (donor Hgb 21.5) was given via the intraperitoneal route.
- At 313/7 weeks PUBS demonstrated a fetal pre-transfusion Hgb 12.4 g/dL. Similar to PUBS #1, given the proximity of this Hgb to the 31-week cutoff of 11.8 g/dL, documented severe fetal anemia on the prior PUBS, and difficulty in obtaining intravascular access, we transfused 30 mL of packed red cells (donor Hgb 20.3) intravascularly raising the post-transfusion Hgb to 14.6 g/dL.
- At 353/7 weeks PUBS demonstrated a pre-transfusion fetal Hgb of 12.9 g/dL. By extrapolation, the 35-week cutoff is 12.6 g/dL. Using the same reasoning as prior PUBS, 45 mL of packed red cells (donor Hgb 22) were transfused intravascularly. The post-transfusion Hgb was 14.6 g/dL.
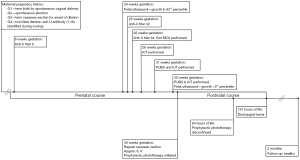
Intrauterine growth restriction was noted on ultrasound 1 day after the last IUT with an estimated fetal weight of 2,189 g (<5%) prompting delivery by repeat cesarean section at 36 weeks with Apgar of 8 and 9. Maternal anti-U titer at this time was found to be 64. Birthweight was 2,205 g, small for gestational age, and physical exam and vital signs were unremarkable with no tachycardia, pallor, or jaundice. Pertinent blood work obtained at 20 minutes of life were: Hgb 16.0 g/dL, reticulocyte 5.6%, blood type A+, direct antiglobulin test 3+, total serum bilirubin 2.5 mg/dL, and direct bilirubin 0.5 mg/dL. The patient fed ad lib and was stable in room air. Early prophylactic phototherapy was initiated and discontinued by 64 hours of age. The patient was discharged home at ~110 hours of life. The discharge Hgb was 16.7 g/dL, Hct 50%, reticulocyte 4.2%, total serum bilirubin of 6.0 mg/dL and direct bilirubin of 0.6 mg/dL. She did not evidence late onset anemia requiring blood transfusion. At 2-month follow-up, the patient was developmentally appropriate. Figure 2 visually depicts the patient’s prenatal and postnatal course.
Please note that all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s mother for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We report a case of severe anti-U-mediated fetal anemia successfully managed by IUT. IUT was performed via both fetal intravascular and intraperitoneal routes using donated U-negative blood. The maternal critical anti-U titer of 64 in our case was lower than those previously reported among IUT-managed cases (8,000, 512, and 256) (10-12). Nevertheless, fetal Hgb levels ranging from 8.9 g/dL at 26 weeks to 12.9 g/dL at 35 weeks characterized the in-utero course. The first IUT was based on the abnormal MCA Doppler being consistently in the severe-anemia range. Subsequent procedures were time-driven based on the predicted rate of donor RBC loss. The decision to transfuse was based on fetal pre-transfusion Hgb but also on the difficulty in both securing fetal intravascular access and in obtaining U-negative blood for both fetus and mother (9).
Postnatally, the infant evidenced a 3+ positive direct antiglobulin test with anti-U eluted from the newborn RBCs. The postnatal course was relatively benign. Our infant, similar to one other IUT case, was managed with phototherapy alone and did not need RBC transfusion during the birth hospitalization or as an outpatient in follow-up (12). In contrast, one other IUT neonate required phototherapy, an exchange transfusion, and three blood transfusions postnatally, while another required phototherapy and a blood transfusion for symptomatic anemia (10,11).
We searched the literature using the general term “anti-U hemolytic disease” in PubMed and identified 14 reports, in English, featuring hemolytic disease of the fetus and newborn (Table 1). Three non-English articles were not included in our review (24-26). The table highlights the potential for severe fetal/neonatal involvement in anti-U hemolytic disease: 4 of the 20 cases merited IUT (including ours), there was one intrauterine fetal demise (IUFD) secondary to erythroblastosis fetalis, and 3 infants who did not undergo IUT required postnatal double volume exchange transfusion (10-16). These cases were characterized by maternal anti-U titers in the 64 to 8,000 range (10,15). The wide range of maternal antibody titers suggest this metric is not a strong predictor of the clinical course of the fetus or newborn. Severe cases resulting in fetal anemia were seen at maternal anti-U titers as low as 64 (15). High titers do not necessarily predict a severe fetal or neonatal course; in three pregnancies with maternal anti-U titers of 4,000 the fetus was unaffected and the newborn was managed with phototherapy alone with no need for postnatal transfusion (10). Only titers below 32 generally resulted in a benign postnatal course of mild jaundice and phototherapy, a conclusion shared by Rana et al. (17). Nevertheless, if anti-U antibody is present, antibody titers should be followed throughout pregnancy and close fetal monitoring is warranted when a critical titer is observed. As reviewed by Adam and Lombaard in 2016, intrapartum management of anti-U hemolytic disease should be in accordance with the management of Rh alloimmunization (12).
Table 1
| Year | Pregnancy# | Maternal ancestry*** | MBT | Maternal antibody titer | Infant DAT | Fetal/neonatal course |
|---|---|---|---|---|---|---|
| 1961, (18) | G6 | Black | B+ | Anti-U: 32 | Positive: anti-U | Uncomplicated, no treatment |
| 1964, (13) | G4 | Black | O+ | Anti-U: 640 | Not obtainable | IUFD at 36 weeks gestation |
| Anti-N: 16 | Postmortem: fetal hydrops secondary to erythroblastosis fetalis | |||||
| 1976, (14) | G4 | Black | – | Anti-U: 128 (8 weeks) → 1,024 (30 weeks) | Positive: anti-U | Phototherapy |
| Double volume exchange transfusion | ||||||
| 1981, (15) | G7 | Black | B− | Anti-U 64 | Positive anti-U and anti-D | Double volume exchange transfusion |
| Anti-D 256 | ||||||
| 1981, (16) | G7 | Nigerian | O+ | Anti-U: 512 | Positive: anti-U | Phototherapy |
| Double volume exchange transfusion | ||||||
| 1982, (19) | G1 | Jamaican | O+ | Anti-U first detected at 16 weeks → 4 (34 weeks) | Positive (no eluate reported) | Phototherapy |
| 1983, (20) | G2 | Black | – | Negative at 15 weeks → anti-U: 32 (at delivery) | Positive: anti-U | Uncomplicated, no treatment |
| G3 | Anti-U 16 | Positive: anti-U | Uncomplicated, no treatment | |||
| 1984, (21) | G2 | Black | AB+ | Positive (no titer reported) | Positive: anti-U | Uncomplicated, no treatment |
| 1998, (10) | G3 | Nigerian | Anti-U: 4 | Negative | Phototherapy | |
| G4 | Anti-U: 16 (7 weeks) → 4,000 (38 weeks) | Positive (no eluate reported) | Phototherapy | |||
| G3 | Zimbabwean | – | Anti-U: 16 (19 weeks) → 128 (29 weeks) → 256 (30 weeks) → 4,000 (35 weeks) | Positive (no eluate reported) | Phototherapy | |
| G4 | Anti-U: 512 (23 weeks) → 2,000 (37 weeks) | Positive (no eluate reported) | Phototherapy | |||
| G2 | Ghanaian | – | Negative (11 weeks) → anti-U: 8,000 (33 weeks) | Positive (no eluate reported) | Phototherapy | |
| Six postnatal blood transfusions | ||||||
| G3 | Anti-U: 1,000 (20 weeks) → 8,000 (32 weeks) | Positive (no eluate reported) | IUT ×4 | |||
| Phototherapy | ||||||
| Double volume exchange transfusion | ||||||
| Three postnatal blood transfusions | ||||||
| 1999, (22) | G2 | Black | A+ | Anti-U: 4 (18 weeks) → 8 (24 weeks) → 16 (34 weeks) | Positive: anti-U | Uncomplicated, no treatment |
| 2003, (23) | G4 | Brazilian** | – | Anti-U: 32 | Positive: anti-U | Phototherapy |
| 2011, (17) | G4 | Nigerian | – | Anti-U: 4 (16 weeks) → 8 (28 weeks) → 32 (36 weeks) → 256 (37 weeks) | Positive (no eluate reported) | Phototherapy |
| 2013, (11) | G4 | Somalian | A+ | Anti U: 256 (15 weeks) | Positive: anti-U | IUT ×4 |
| Phototherapy | ||||||
| One postnatal blood transfusion | ||||||
| 2016, (12) | G1 | Black | O− | Anti-U: 512 (25 weeks) | Positive: anti-U | IUT ×2 |
| Anti-D: not detected | Uncomplicated postnatal course, no treatment | |||||
| 2020* | G5 | Black | A+ | 8 (8 weeks) → 32 (25 weeks) → 64 (26 weeks) | Positive: anti-U | IUT ×4 |
| Phototherapy |
*, current case report. **, in this reference from Brazil, mother is identified as “brown”. This term is often used in Brazil for individuals of admixed European, West African, and Amerindian descent. ***, when specified, an individual’s country of origin was used for maternal ancestry. However, some studies pre-dating the 2000s used derogatory and pejorative rhetoric when describing a patient’s ancestry, race, or ethnicity. As such, the term Black is broadly used to convey ancestry by way of referring to a shared history, identity, or community which includes individuals within Africa or part of the African diaspora. G, gravida; MBT, maternal blood type; DAT, direct antiglobulin test; GA, gestational age; IUT, intrauterine transfusion; IUFD, intrauterine fetal demise.
Conclusions
Anti-U hemolytic disease of the fetus and newborn is a rare, but potentially serious condition and should be managed in accordance with Rh alloimmunization guidelines. Antibody titers are inconsistently associated with clinical severity of disease. Surveillance with MCA Doppler and IUT with donated U-negative blood have shown promising outcomes.
Acknowledgments
We would like to acknowledge Vivek Ashok, MD for his time and dedication towards critically reviewing the contents, verbiage, and semantics of this manuscript to eliminate discriminatory and pejorative language that promotes the practice of race-based medicine and opposes health equity.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/pm-21-72
Peer Review File: Available at https://dx.doi.org/10.21037/pm-21-72
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pm-21-72). JFW reports serving as a consultant in medicolegal cases associated with kernicterus. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s mother for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wiener AS, Unger LJ, Gordon EB. Fatal hemolytic transfusion reaction caused by sensitization to a new blood factor U: report of a case. J Am Med Assoc 1953;153:1444-6. [Crossref] [PubMed]
- Wiener AS, Unger LJ, Cohen L. Distribution and heredity of blood factor U. Science 1954;119:734-5. [Crossref] [PubMed]
- Greenwalt TJ, Sasaki T, Sanger R, et al. An Allele of the S(s) Blood Group Genes. Proc Natl Acad Sci U S A 1954;40:1126-9. [Crossref] [PubMed]
- Hoekstra A, Albert AP, Newell GA, et al. S--s--U-- phenotype in South African Negroes. Vox Sang 1975;29:214-6. [Crossref] [PubMed]
- Reid ME. MNS blood group system: a review. Immunohematology 2009;25:95-101. [Crossref] [PubMed]
- Anstee DJ. Blood group-active surface molecules of the human red blood cell. Vox Sang 1990;58:1-20. [Crossref] [PubMed]
- Roush GR, Rosenthal NS, Gerson SL, et al. An unusual case of autoimmune hemolytic anemia with reticulocytopenia, erythroid dysplasia, and an IgG2 autoanti-U. Transfusion 1996;36:575-80. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013;2:38-43. [Crossref] [PubMed]
- Nicolaides KH, Soothill PW, Clewell WH, et al. Fetal haemoglobin measurement in the assessment of red cell isoimmunisation. Lancet 1988;1:1073-5. [Crossref] [PubMed]
- Smith G, Knott P, Rissik J, et al. Anti-U and haemolytic disease of the fetus and newborn. Br J Obstet Gynaecol 1998;105:1318-21. [Crossref] [PubMed]
- Strindberg J, Lundahl J, Ajne G. Hemolytic disease of the fetus and newborn owing to anti-U, successfully treated with repeated intrauterine transfusions. Immunohematology 2013;29:51-4. [Crossref] [PubMed]
- Adam S, Lombaard H. Autologous intrauterine transfusion in a case of anti-U. Transfusion 2016;56:3029-32. [Crossref] [PubMed]
- Buerki U, Degnan TJ, Rosenfield RE. Stillbirth due to anti-U. Vox Sang 1964;9:209-11. [Crossref] [PubMed]
- Austin TK, Finklestein J, Okada DM, et al. Letter: Hemolytic disease of newborn infant due to anti-U. J Pediatr 1976;89:330-1. [Crossref] [PubMed]
- Gottschall JL. Hemolytic disease of the newborn with anti-U. Transfusion 1981;21:230-2. [Crossref] [PubMed]
- Dhandsa N, Williams M, Joss V, et al. Haemolytic disease of the newborn caused by anti-U. Lancet 1981;2:1232. [Crossref] [PubMed]
- Rana R, De Graaf F, Kumaranayakan P. Anti-U antibody in pregnancy: a rare antibody causing hemolytic disease. Acta Obstet Gynecol Scand 2011;90:555. [Crossref] [PubMed]
- Afonso JF, De Alvarez RR. Maternal isosensitization to the red cell antigen U. Am J Obstet Gynecol 1961;81:45-8. [Crossref] [PubMed]
- Tuck SM, Studd JW, White JM. Sickle cell disease in pregnancy complicated by anti-U antibody. Case report. Br J Obstet Gynaecol 1982;89:91-2. [Crossref] [PubMed]
- Dopp SL, Isham BE. Anti-U and hemolytic disease of the newborn. Transfusion 1983;23:273-4. [Crossref] [PubMed]
- Turner RJ, Holder WT, McCord DL. Isoimmunization with anti-U antibody. J Natl Med Assoc 1984;76:277-8,283. [PubMed]
- Parsons DDS. Anti-U antibodies during pregnancy with mild hemolytic disease of the newborn. Lab Med 1999;30:771-5. [Crossref]
- Novaretti MC, Jens E, Pagliarini T, et al. Hemolytic disease of the newborn due to anti-U. Rev Hosp Clin Fac Med Sao Paulo 2003;58:320-3. [Crossref] [PubMed]
- Magaud JP, Jouvenceaux A, Bertrix F, et al. Perinatal hemolytic disease due to incompatibility in the U system (author's transl). Arch Fr Pediatr 1981;38:769-71. [PubMed]
- Castel A, Drejer GF, Verwey RA. Hemolytic disease in a newborn infant caused by rare maternal anti-erythrocyte antibodies and exchange transfusion with maternal blood frozen earlier. Ned Tijdschr Geneeskd 1993;137:2713-5. [PubMed]
- García Bueno MJ, Fernández Jiménez B, Muñiz-Díaz E, et al. Autologous transfusion in a pregnant woman with anti-U and anti-He (Henshaw) antibodies. Med Clin (Barc) 2005;124:679. [PubMed]
Cite this article as: Li JC, Hwang MS, Emery SP, Watchko JF, Ibrahim JW. Anti-U hemolytic disease of the fetus and newborn managed by multiple intrauterine transfusions: a case report and review of the literature. Pediatr Med 2021;4:38.

