
Narrative review of bilirubin measurement and binding
Introduction
The primary goal of the evaluation and management of unconjugated hyperbilirubinemia in premature and term infants is to prevent bilirubin-induced neurological dysfunction (BIND), a spectrum of neurodevelopmental disorders, including but not limited to central apnea, auditory disorders, communication and language disorders, autism, attention deficit hyperactivity disorder (ADHD), upward gaze palsy, and athetoid cerebral palsy (1). To-date, total serum bilirubin (TSB) concentration remains the primary bilirubin biochemical measure used to evaluate and manage unconjugated hyperbilirubinemia in premature and term infants. However, the current TSB threshold for phototherapy and/or exchange transfusion is a poor predictor of BIND (2-8). This is not surprising because of the complex underlying pathogenesis of BIND (9). Most importantly, bilirubin-albumin binding limits the use of TSB as an indicator of the amount of bilirubin in vascular and extravascular space, the magnitude of ongoing bilirubin production/excretion mismatch, and the overall risk of bilirubin toxicity. This is supported by mounting evidence in the literature that non-albumin bound (unbound), also known as free bilirubin (Bf), resulting from altered bilirubin-albumin binding is a more sensitive and specific biochemical measure of acute and chronic bilirubin-induced neurotoxicity than TSB in preterm and term infants. Thus, measurement of bilirubin and bilirubin binding are critical for the assessment of the risk of BIND. Our objectives for this narrative review are to: (I) provide current literature on bilirubin and bilirubin binding in premature and term infants with unconjugated hyperbilirubinemia and (II) inform promising newer technologies currently in development as point-of-care (POC) methods for bilirubin and binding measurement.
I present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/pm-21-15).
Methods
In order to assimilate literature and synthesize key findings for the objectives of this narrative review, we searched published literature in the English language using PubMed with the following keywords: bilirubin, bilirubin-albumin binding affinity, structure, properties, bilirubin binding capacity, unbound bilirubin, free bilirubin, TSB, bilirubin albumin molar ratio (BAMR), BIND, kernicterus, technologies, methods, neonates, and infants. We also considered abstracts of clinical studies presented at the Pediatric Academic Society Meeting in the past 15 years.
Discussion
Bilirubin and bilirubin binding
Properties of bilirubin and bilirubin binding to albumin
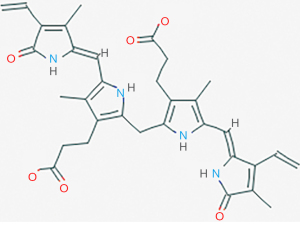
Bilirubin binding to albumin is based on the properties of bilirubin and albumin. The native form of unconjugated bilirubin (a 4Z, 15Z-IXα isomer) has two rigid dipyrrole units internally hydrogen bonded to each other so that no ionizable groups are exposed, allowing it to be almost insoluble in water and form harmful dimers and higher aggregates (10,11) (see Figure 1). The solubility of unconjugated bilirubin increases with increasing pH due to ionization of the acidic groups on its molecule (13,14). The hydrophobic native form of unbound, unconjugated bilirubin (a 4Z, 15Z-IXα isomer) can readily cross biomembranes and is neurotoxic (15,16). It is not yet known if other isomers of bilirubin are neurotoxic (12).
Bilirubin-albumin binding capacity and affinity
Bilirubin, a product of hemoglobin degradation, exists in the blood mostly bound to albumin. Bilirubin-albumin binding depends on the concentrations of bilirubin and albumin, as well as the binding affinity for bilirubin. When bound, bilirubin is unable to cross the intact blood-brain barrier to cause BIND (2,17).
Albumin is a highly abundant serum protein and allows the transport of bilirubin to the liver where bilirubin dissociates from albumin and is conjugated by the hepatic cells. There are at least two types of binding sites for bilirubin on albumin. The strongest and most specific site has a very high binding affinity for bilirubin, approximately 1.4×107 mol/L at 37 °C. Secondary sites have at least a ten-fold lower binding affinity for bilirubin (11,18,19).
The fraction of free or unbound bilirubin (Bf) significantly increases as the TSB concentration exceeds the binding capacity of albumin or when bilirubin-albumin binding affinity decreases (10). There are several factors that may decrease the binding affinity of albumin to bilirubin, including increasing temperature, sepsis, acidosis, hypoxia, hypothermia, free fatty acids (FFA) or albumin binding drugs (2,10,20,21).
Strong experimental evidence suggests that due to both the abundance of albumin and its high affinity for bilirubin, the level of Bf in the serum is largely controlled by the albumin binding capacity in healthy neonates (10,19,22). The bilirubin binding capacity at the high affinity binding site can be represented by the following equilibrium equation:
R denotes the concentration of albumin with vacant high-affinity sites and B denotes the concentration of albumin with bilirubin bound to high-affinity albumin sites. The total amount of albumin available to strongly bind bilirubin (R + B) at the high affinity binding site constitutes the bilirubin binding capacity, or C. One major limitation is that the relationship does not account for bilirubin binding to low affinity sites. Several different techniques were used in the past to measure total and reserve bilirubin binding capacity and provide an indirect measurement of Bf, but each technique has limitations (17). Of note, there are no tests for determining total bilirubin binding capacity (the total bilirubin level at which all plasma bilirubin binding sites are occupied) (17,23).
Bilirubin-albumin binding is a reversible and dynamic process in which bilirubin is continuously binding onto and dissociating off of albumin at normal serum pH and temperature (24,25). When there is poor bilirubin binding, elevated Bf exits into the tissues, including the central nervous system, at a rate that is proportional to the serum Bf concentration. The likelihood of BIND increases with increasing Bf concentrations in and around cells of the central nervous system. As bilirubin exits the vascular system and enters the tissues, more bilirubin is dissociating from the albumin bound pool present in the vascular system. The relationship between serum Bf, TSB, and albumin concentrations is explained by the law of mass action and is characterized by an affinity or binding constant, K, as shown in Eq. [2]:
The law of mass action equation clearly indicates that Bf is inversely proportional to albumin and its intrinsic ability to bind bilirubin (K), as shown in Eq. [3]
It also underscores the significance of evaluating bilirubin-albumin binding in addition to TSB to estimate Bf and indirectly determine the risk for BIND (25). This can be further explained by considering the changes in unconjugated bilirubin load (imbalance with bilirubin production and elimination) with or without changes in K or albumin concentration. The imbalance between unconjugated bilirubin production and elimination at constant albumin concentration and K will increase TSB. However, once the albumin is saturated with unconjugated bilirubin, the incremental increase in TSB with increasing bilirubin load decreases, while the incremental increase in Bf will accelerate (25). Thus, an increased but stable TSB should not be considered safe. Alternatively, increases in albumin concentration and K at constant unconjugated bilirubin production and elimination will increase TSB (mainly bound bilirubin), but not Bf and the risk of BIND. Thus, variations in albumin concentration and K undermine the ability of TSB but not that of Bf to predict BIND (25).
A bilirubin binding panel (BBP) has recently been proposed to measure bilirubin binding in neonates and to generate Bf population parameters for threshold TSB at which phototherapy and/or exchange transfusion is indicated (26). For the BBP, two empiric constants: maximum TSB (TSBmax) and its corresponding equilibrium association constant (K) are required in addition to TSB and Bf. TSBmax is an empirical mass action upper limit of TSB in a sample that is used with K to calculate Bf at any TSB < TSBmax {Eq. [3]}. TSBmax and K are necessary to calculate Bf at the threshold TSB level since the TSB of a sample is rarely the threshold TSB. Additionally, TSB is the bound bilirubin concentration because Bf is orders of magnitude less than TSB (µg/dL versus mg/dL) and bound bilirubin = TSB – Bf ≅ TSB. Unoccupied binding sites, or R, in the derivation of Eq. [2] equals TSBmax – TSB. Therefore, K {described above in Eq. [2]} is as follows:
TSBmax is a new variable introduced to eliminate the misconception that only the high affinity binding site is clinically relevant in bilirubin binding. Both bilirubin binding sites are clinically relevant as was confirmed in a recent study (27). An empirical TSBmax can readily be obtained from TSB and Bf measured before and after sample bilirubin enrichment. Enriching samples with bilirubin to TSB above the relevant TSB thresholds and re-measuring TSB and Bf to obtain TSBmax and K for accurate estimates of Bf at threshold TSB < TSBmax requires a small sample volume. Once Bf population parameters are derived, the BBP can be used to help determine how best to manage a neonate that has reached a consensus-based TSB threshold for phototherapy and/or exchange transfusion (27).For example, a 29-week GA infant has a phototherapy TSB threshold of 8 mg/dL. Assume the median Bf for the population at TSB =8 mg/dL is 0.60 µg/dL with the 25th percentile 0.45 µg/dL and 75th percentile 0.75 µg/dL. If the infant’s Bf is >0.60 µg/dL phototherapy is indicated, but if it is >0.75 µg/dL there is significant concern for an increased risk of BIND as binding is relatively poor compared to other infants at this gestational age (GA). Once available, the clinical use of bilirubin binding may improve existing clinical practice involving consensus-based TSB thresholds for management of hyperbilirubinemia.
Bilirubin-albumin binding as a function of gestational age and postnatal age
Bilirubin binding has been studied as a function of gestational and postnatal age using different methods. During the first postnatal week, premature infants are at increased risk of BIND at lower concentrations of TSB than term infants (2,10). The serum albumin levels and bilirubin binding capacity (BBC) is lower in premature infants compared to their term counterparts (10,28-30) and partly explains the increased risk of BIND. Cashore et al. reported that the BBC remains unchanged during the first ten days of life in both healthy term and preterm infants using the Sephadex method, but the BBC is highly variable in all sick infants (30). In a recent study, blood samples taken at five days of life demonstrated that the BBC at a high-affinity binding site improves with increasing GA in <31 weeks GA infants. However, these findings were based on a single peroxidase concentration with several assumptions made about the binding of bilirubin to albumin (23,31). Similar findings were recently reported by Lamola et al., who found a positive correlation between GA and BBC measured by two different types of the hematofluorometric instrument (which assumes a single non-interactive, high-affinity binding site on albumin) in three different cohorts of preterm and term infants (32). The BBC increased by 1 mg/dL/week with increasing GA at the high affinity binding sites (32).
The literature on bilirubin binding affinity (K) is scant. One large prospective study, using the modified peroxidase method, found that in premature infants ≤30 weeks GA, the bilirubin binding affinity was variable, but showed an overall decrease during the first week of life and was primarily responsible for elevated Bf in premature infants ≤30 weeks GA (33). Additionally, due to an uncoupling relationship between Bf and TSB, as TSB decreases with phototherapy, Bf increases in premature infants ≤30 weeks GA (33). This phenomenon is highly indicative of risk factors such as sepsis, acidosis, hypothermia, hypoxia, or bilirubin displacers such as FFA and albumin binding drugs (2,20,21,33). Contrastingly, in premature infants >30 weeks GA, the binding affinity gradually improves with postnatal age during the first postnatal week. In term infants, binding affinity measured indirectly using the Sephadex gel filtration method increases as early as the third postnatal day of life and continues to increase, reaching adult serum levels by five months of age (34). The improvement in binding affinity is thought to be due to maturational changes in the ability of albumin to bind bilirubin with increasing postnatal age.
Clinical risk factors associated with bilirubin-albumin binding
The risk factors that are thought to increase bilirubin-induced neurotoxicity occur either through the disruption of bilirubin-albumin binding and/or by facilitation of Bf entry into the brain (2), see Table 1. For example, Cashore et al. found that reserve albumin concentration determined by dialysis with 14C-monoacetyldiaminodiphenylsulfone was decreased and the bilirubin toxicity index was increased in the presence of clinical risk factors such as acidosis, hypoxia, or sepsis, increasing the risk of BIND (35). Meisel et al. evaluated how acid-base status influences bilirubin-albumin binding in low birthweight infants between three to eight days of life. They found a significant correlation between metabolic acidosis and the bilirubin to albumin reserve ratio as well as the bilirubin toxicity index (28). Additionally, shifting to a metabolic acidosis state (with a base deficit of 10) showed a decrease in bilirubin-albumin binding and doubled the toxic potential of Bf (28). Alternatively, Kozuki et al. found that correcting metabolic acidosis during the first day of life in late preterm infants resulted in an improvement of the bilirubin-albumin binding affinity and decreased levels of Bf (36).
Table 1
| 1. Prematurity |
| 2. Low birth weight |
| 3. Hypoalbuminemia |
| 4. Sepsis |
| 5. Metabolic acidosis |
| 6. Hypoxia |
| 7. Hypothermia |
| 8. Free fatty acids |
| 9. High protein binding drugs (e.g., sulfa drugs, ceftriaxone, ibuprofen) |
Neonatal sepsis causes both a decrease in albumin production and an increase in FFA concentration, a known displacer of bilirubin, although the mechanism remains unclear. These factors are thought to disrupt bilirubin-albumin binding (10,37,38). Both hypoxia and hypothermia can also cause an increase in FFA concentration. One small prospective study involving preterm infants showed that prolonged hypoxia, hypothermia, and acidosis occurring prior to the peak of Bf levels significantly increased the risk of BIND (39).
Although some evidence can be derived from these observational studies about the roles that clinical risk factors play in the pathogenesis of BIND more studies are needed to confirm. Overall, many experts would recommend aggressive management and treatment of hyperbilirubinemia in the presence of one or more clinical risk factor (Table 1).
Exogenous drugs and how it affects bilirubin-albumin binding
Several therapeutic drugs and preservatives used in their preparation have been shown to displace bilirubin from albumin binding sites. The most well-known potent bilirubin displacers include sulfa drugs, ceftriaxone, and ibuprofen (2). These drugs should be avoided as long as there is a risk for bilirubin-induced neurotoxicity in premature and term infants (2). With regards to Ibuprofen, the evidence suggests that in premature infants clinically significant displacement is unlikely if intravenous (IV) ibuprofen is used at the recommended dosage (such as for the treatment of a patent ductus arteriosus) after the first two postnatal days and with a bilirubin to albumin molar ratio (BAMR) <0.5. Caution should be taken when using IV ibuprofen in those infants with a BAMR >0.5 and during the first two postnatal days when the plasma levels of ibuprofen may be higher (40-46). Ceftriaxone use has been cautioned against in the presence of unconjugated hyperbilirubinemia because of its potential for displacing bilirubin from albumin resulting in elevated free bilirubin concentration (47,48). However, in a recent clinical trial, IV ceftriaxone was not associated with bilirubin displacing effects in term infants with mild unconjugated hyperbilirubinemia (TSB ≤10 mg/dL) during the first postnatal week (49); however, it should be avoided in term infants with TSB >10 mg/dL.
Additionally, almost all premature infants receive total parenteral nutrition in the first several days of life, including addition of intravenous lipid (IL). IL intake in premature infants may result in elevated levels of FFA and the clearance of FFA is slower in premature infants compared to their term counterparts. This is important because FFA can bind to albumin at three different sites, one of which is the high affinity binding site that bilirubin uses to bind albumin. When the FFA to albumin molar ratio is greater than or equal to 4:1, FFA competes with bilirubin resulting in displacement of bilirubin from albumin and increased levels of Bf in the serum. The current evidence suggests that only infants who are ≤28 weeks GA during the first post-natal week of life are affected by this decrease in bilirubin-albumin binding affinity caused by IL intake (50-52). Therefore, IL intake should be limited in these extremely premature infants during the first post-natal week of life when unconjugated hyperbilirubinemia is most common (32,50-53).
Association of BAMR with bind
The consensus-based guidelines for the management of hyperbilirubinemia in term and preterm infants recommend measuring serum albumin. The utility of this is based on existing evidence, especially in the absence of clinical methods for bilirubin binding measurement. Scheidt et al. reported no significant association of TSB or BAMR with neurodevelopmental outcomes, including cerebral palsy and intellectual disability, after controlling for neonatal risk factors (3). Similarly, Cashore et al. in a small retrospective study involving premature infants reported no significant association between TSB or BAMR and autopsy proven kernicterus (35). However, most others have reported the possible usefulness of BAMR in premature infants. Kim et al. and Ritter et al. independently reported that calculated peak BAMR was higher in infants with kernicterus compared to those without kernicterus (39,54). Amin et al. demonstrated that peak BAMR, but not peak TSB, was significantly higher in infants with abnormal auditory brainstem response (ABR) maturation compared to infants with normal ABR maturation in a subset of forty-five premature infants in whom Bf was measured (55).
A multicenter randomized trial in the Netherlands found that the use of BAMR in addition to TSB was associated with reduction in mortality compared to TSB alone in a subgroup of infants with a birth weight >1,000 g (56).A case series of five sick premature infants who were demonstrated to have MRI findings of kernicterus with peak TSB concentrations ranging from 8.7 to 11.9 mg/dL but with relatively low serum albumin concentrations ranging from 1.4 to 2.1 g/dL (BAMR >0.47) further emphasizes the importance of measurement of BAMR in premature infants (57). Furthermore, in late preterm and term infants, BAMR was a better predictor than TSB of bilirubin-induced auditory toxicity (58). Overall, the limited evidence favors measurement of serum albumin and BAMR in addition to TSB for the management of unconjugated hyperbilirubinemia in premature and term infants.
Association of bilirubin binding and free bilirubin with BIND
Both indirect and direct methods of Bf measurement have been used to evaluate an association of Bf with BIND. In earlier studies, indirect methods, such as the Sephadex gel filtration method for bilirubin binding capacity, dye binding method for reserve binding capacity, and salicylate saturation index for bilirubin binding affinity, were used (17). Using indirect method, significant association was reported between low bilirubin reserve binding capacity and abnormal communication and language skills at age seven in 30–41 weeks GA infants with unconjugated hyperbilirubinemia (59).
Cashore et al. reported higher Bf concentrations measured using the peroxidase method on five premature infants with autopsy proven kernicterus compared to eight premature infants without kernicterus (35) (Table 2). Similarly, Ritter et al. reported a higher Bf concentration in seven infants with kernicterus compared to twenty-three infants without kernicterus in a prospective study (39) (Table 2).Several studies have recently corroborated the usefulness of Bf as a predictor of BIND in premature and term infants using the modified peroxidase method (55,58,60-66). Nakamura et al. in a prospective study reported that both peak TSB and peak Bf were significantly higher in infants with kernicterus compared to those without kernicterus (60).
Table 2
| Study | GA or birth weight | Mean free bilirubin (µg/dL) | ||
|---|---|---|---|---|
| No KI or normal ABR | ABR changes | Overt KI | ||
| Ritter, 1982, (39) | 26–32 weeks GA | 0.65±0.05 | Not done | 1.1±0.26 |
| Cashore, 1983, (35) | ≤1,500 g | 0.76±0.58 | Not done | 1.6±0.52 |
| Nakamura, 1992, (60) | 1,500–2,499 g; <1,500 g | 0.54±0.2 | Not done | 1.3±0.24 |
| 0.46±0.17 | 1.13±0.34 | |||
| Amin, 2001, (55) | 28–32 weeks GA | 0.40±0.15 | 0.62±0.2 | None |
| Ahlfors, 2009, (61) | 24–41 weeks GA | 0.93±0.70 | 1.76±1.31 | None |
| Amin, 2017, (62) | ≥34 weeks GA with severe jaundice | 1.44±0.94 | 4.74±3.92 | None |
| Amin, 2016, (63) | ≥34 weeks GA with severe jaundice | 1.7±0.8 | 4.2±1.7 | None |
| Amin, 2017, (58) | ≥34 weeks GA with severe jaundice | 1.24±0.74 | 3.37±2.92 | None |
GA, gestational age; KI, kernicterus; ABR, auditory brainstem response.
In recent longitudinal studies involving late preterm and term infants with severe jaundice, peak Bf, but not peak TSB, was shown to be associated with acute and chronic bilirubin-induced auditory toxicity (58,62,63) (Table 2). Amin et al. in a small prospective study involving 45, 28–32 weeks GA infants reported that peak Bf, but not peak TSB, was significantly higher in infants with abnormal ABR maturation compared to infants with normal ABR maturation (55). In addition, in a retrospective study, premature infants with abnormal ABR maturation had more concurrent apnea and bradycardia and required more prolonged respiratory support and methylxanthine therapy (64). This was corroborated in a prospective study which demonstrated that central apnea, a manifestation of bilirubin-induced brainstem dysfunction, was more closely associated with serum Bf levels than TSB in premature infants (65). Ahlfors et al. in a retrospective study involving 24–42 weeks GA infants reported that Bf (odds ratio 3.3, 95% CI: 1.8 to 6.1) and not TSB (odds ratio 1.04, 95% CI: 0.94 to 1.16) was strongly associated with abnormal automated ABR screening tests performed prior to discharge (61). In another longitudinal study, an increasing serum Bf level measured once at approximately five days of life using the peroxidase method in extremely low birth weight infants was associated with a higher risk of death or adverse neurodevelopmental outcomes irrespective of clinical status (66). In summary, there is ample evidence to support the importance of Bf measurement in premature and term infants.
Methods of bilirubin measurement
Total bilirubin determination
Of newborns, greater than eighty percent develop visual jaundice in the first few days of life. Bilirubin levels typically peak in neonates at approximately ninety-six hours of life long after most infants are discharged home. The American Academy of Pediatrics (AAP) recommends that infants discharged before 72hours of life should be seen by a healthcare provider within the next 48 to 70 hours post-discharge to assess for jaundice. It is also routine practice for all infants to have their bilirubin measured prior to hospital discharge. Ample evidence suggests that even experienced health care providers cannot accurately estimate the severity of jaundice based purely on visual assessment (67), therefore objective methods are recommended for bilirubin measurement. Below are available methods for measuring bilirubin.
Total serum/plasma bilirubin measurement
Bilirubin can be measured in body fluids by a variety of analytical methods including high performance liquid chromatography (HPLC), enzymatic methods (bilirubin oxidase, vanadate oxidase methods), electrophoresis, and direct spectrophotometry (68). HPLC, which effects separation and quantitation of the four bilirubin fractions, is the gold standard method of choice, but not easily available for routine use. Clinical chemistry laboratories typically utilize automated clinical chemistry assays. The diazo method, originally developed by Ehrlich in 1883, is the most widely used method for measurement of different forms of bilirubin. Conjugated bilirubin reacts directly with the diazo reagent (thus the designation of conjugated bilirubin as “directly reacting” or “direct” bilirubin). In the automated diazo method, serum or plasma is incubated with diazo reagent at an acidic pH of approximately 2.0 to form a diazonium salt. The resulting product reacts with bilirubin to form isomers of azobilirubin. In direct bilirubin assays, the conjugated bilirubin is the predominant form converted by the diazotized sulfanilic acid (68). However, approximately 5% of unconjugated bilirubin may react as well which could obscure the clinical diagnosis. The intensity of the red color of azobilirubin is measured photometrically at approximately 600 nm and is proportional to the direct bilirubin concentration. Unconjugated bilirubin reacts with diazo reagents with the addition of caffeine, sodium benzoate, or methanol, thus allowing for the determination of the TSB concentration. The difference between TSB concentration and direct bilirubin concentration allows for the estimation of the unconjugated bilirubin concentration or “indirect” bilirubin.
The automated diazo methods are inexpensive, but they have limitations, including interference by low levels of hemolysis and by lipemia or paraproteins. Additionally, interlaboratory variability of bilirubin measurements has been reported by Vreman and van Imhoff et al. (69,70). The accurate and precise measurement of total bilirubin is critical in the evaluation and management of neonatal jaundice. The availability of a Standard Reference Method (SRM) for bilirubin and reliable calibrators has improved the precision and accuracy of TSB measurement by analytic methods. It also compares the results against those obtained by the reference method. To maintain high standards, the performance of laboratories participating in the CAP Neonatal Bilirubin Surveys is graded on the basis of results obtained by the reference method (71).
Transcutaneous bilirubin (TcB) measurement
As an alternative to TSB, TcB measurement is widely used as a screening tool for neonatal jaundice. It was first developed in the early 1980’s to measure total bilirubin levels and minimize the inconvenience of frequent blood draws, which are associated with pain, infection and anemia, especially for premature infants. The equipment used was initially developed to correlate with the intensity of the yellow skin color. However, many factors were found to interfere with the accuracy of this measurement including levels of melanin, hemoglobin, and connective tissue (72). Over the past 20 years, a new generation of devices has been produced to measure TcB levels. These new devices are based on microspectrophotometry, allowing the optical density of bilirubin to be measured more accurately while excluding factors that interfere with bilirubin measurement (73). With this technique, a good correlation between transcutaneous and serum bilirubin has been appreciated even in multiracial populations (74). TcB measurements are most often done in the in-patient setting, prior to hospital discharge in near term and term infants. Several factors influence TcB measurements including GA, postnatal age, skin color/ethnicity, measurement site, TcB devices and their algorithm (75-78).
In term infants, several studies have shown good correlation between TcB and TSB levels prior to the initiation of phototherapy. Based on this evidence, the AAP recommends the use of TcB devices for the screening of jaundice in ≥35 weeks GA infants (79). The caveat is that TcB underestimates TSB at TSB ≥15 mg/dL and in newborns with birth weights <2,500 g during the first 48 hours after birth. This is particularly important as failing to identify a newborn with significant hyperbilirubinemia could lead to a delay in the initiation of appropriate evaluation and management (80,81). There are also some concerns about the usefulness of TcB during phototherapy. In a recent meta-analysis of fourteen studies to evaluate the effect of phototherapy on the reliability of TcB devices in near term and term infants, the pooled estimates of correlation coefficients (r) during phototherapy were: covered sites 0.71 (95% CI: 0.64–0.77), uncovered sites 0.65 (95% CI: 0.55–0.74), forehead 0.70 (95% CI: 0.64–0.75) and sternum 0.64 (95% CI: 0.43–0.77), suggesting a moderate correlation between TcB and TSB during phototherapy (82).
In premature infants, several studies have reported a good correlation but an underestimation of TSB by TcB measurements taken on the forehead, sternum, abdomen or hip bone. A recent comprehensive meta-analysis including twenty-eight studies reported pooled estimates of r=0.82 (95% CI: 0.78–0.85) in random effect and r=0.803 (95% CI: 0.78–0.81) in fixed effect models (83). The study reported that the forehead and sternum were comparable with r=0.82 (95% CI: 0.78–0.85) and that the estimated pooled r of 0.83 was also comparable between two devices, JM103® and Bilicheck® (83). The correlation between TSB and TcB levels is significantly weaker for TcB measurements on uncovered skin compared to covered skin. Hulzebos et al., found that TcB levels measured using a JM-103® instrument on covered hipbones of infants <32 weeks GA before, during, and after phototherapy showed a good correlation and agreement with TSB levels. Their data also showed that TcB persistently underestimated the TSB level and that phototherapy further affected this underestimation up to forty-eight hours after phototherapy was discontinued. A novel formula to correct this underestimation was applied: add 50 µmol/L to the measured TcB value and use 70% of the TSB phototherapy treatment threshold. Using this formula, blood draws were reduced by 40% and there was minimal risk of missing infants <32 weeks GA with significant indirect hyperbilirubinemia (84).
In summary, TcB measurements have proved useful for screening infants ≥35 weeks GA for hyperbilirubinemia. Several recent studies have also demonstrated the utility of TcB as a screening tool for infants >28 weeks GA, further reducing the need for frequent blood draws (83,85-87). Attention should be given to the brand of bilirubinometer used as some correlate better with TSB levels; for example, evidence suggests that the BiliCheck® and Minolta-JM 103® perform better than the BiliMed® device (88).
The Bilistick System, a POC method for measuring TSB level in whole blood (capillary or venous) was recently developed for low-resource countries (89-91). The method involves inserting a Bilistick Test Strip into the Bilistick Reader followed by loading a small drop of blood onto it. The test can be performed at home by trained staff and the turn-over time is 2 minutes. It was recently evaluated for diagnostic performance against TSB measured by laboratory method in a large number of infants from Egypt, Indonesia, Vietnam, and Nigeria and was found to have 92.5% positive predictive value and 92.8% negative predictive value (89). The screening performance is also comparable to laboratory TSB but generally underestimates laboratory measured TSB levels (90). In a study involving newborn infants from India, Bilistick was accurate only in 54.5% infants in measuring TSB within ±2 mg/dL difference of TSB measured by spectrophotometry (91). More studies are required to validate its screening and diagnostic performance in additional racial and ethnic groups.
Smartphone applications for bilirubin measurement
Emerging evidence indicates that smartphone can be used for measuring infants with jaundice. Some of these smart phone techniques require further refinement and are not yet ready for clinical use (92). The BiliCam app is a fairly new technology that is capable of analyzing digital images of newborns using a smartphone, such as the iPhones. The app is designed to obtain images of the skin overlying the infant’s sternum in a standardized way and then transmits the image data to a computer server via the Internet for analysis (67). As described by Taylor et al., a color calibration card (6 cm ×6 cm modified Macbeth Color Checker in the shape of a hollow square) is adhered to the newborn’s sternum and used in the process of collecting the BiliCam images. The calibration cards help account for variations in lighting and facilitate both image capture and data extraction. The cards are printed on specially coated paper to help reduce glare and the color accuracy is checked during the printing process to ensure stability between batches. When initiating the app, a red square appears on the smartphone screen. When the square is aligned with the color calibration card and lighting is adequate, the app automatically captures images from the smartphone camera both with and without a flash. Flash and non-flash images are obtained at three distances from the newborn, totaling six images, and are then sent to the computer server. This process of obtaining photos takes approximately less than one minute. This method of estimating bilirubin values in newborns ≥35 weeks gestation and <7 days of life was found to have similar accuracy to that of TcB measurements in the study conducted by Taylor et al. (67). Of note, Asian American neonates showed less of a correlation between BiliCam bilirubin and TSB measurements. BiliCam was highly sensitive in identifying newborns with a TSB level of ≥17 mg/dL. Therefore, using a threshold value of 13 mg/dL to define a positive BiliCam result could eliminate unnecessary blood draws for the majority of infants screened. The overall conclusion is that BiliCam, though it does not have adequate accuracy to serve as a solitary method for assessing jaundice in newborns, is a comparable screening device to TcB meters when determining which neonates require a TSB level (67). Though this technology is inexpensive and widely available, it is not validated in infants <35 weeks GA.
Recently, a novel smartphone method to screen for neonatal jaundice by imaging the sclera was proposed to reduce the confounding effect of skin pigmentation and ambient light. The Scleral-Conjunctival Bilirubin (SCB) correlated well with TSB (r=0.75) in a small study involving thirty-seven infants and the results were comparable with transcutaneous bilirubinometers (93).
Direct methods of free bilirubin determination
Enzyme based methods
- Bilirubin oxidase methods. A technique using a bilirubin oxidase/fluorescein conjugate differentially assay Bf, albumin bound bilirubin, and conjugated bilirubin in serum. Fluorescence of the modified enzyme exhibits changes with time depending on the type and concentration of bilirubin. While this method is useful in obtaining a simultaneous estimate of the three types of bilirubin, it is not sufficiently accurate or sensitive to be used in Bf assays in clinical samples. Additionally, this method is affected by the presence of serum hemoglobin and lipid emulsion (17,94).
- Peroxidase method using UB analyzer. The method using an appropriate concentration of the peroxidase enzyme for serum TSB and Bf measurement was first introduced in 1974 by Jacobsen and Wennberg and is currently the most commonly used method for Bf measurement (17). Bf and not bound bilirubin react to peroxidase and therefore horse radish peroxidase catalyzed oxidation rate of Bf derived from the time course of the diminishing bilirubin color is used to determine the concentration of Bf in the serum (17). An FDA approved standardized and dedicated spectrophotometer (UB-A1 Analyzer, Arrows Co Ltd) for Bf measurement using the peroxidase method is widely used for clinical research (25). In the original peroxidase method, a 42-fold dilution with phosphate buffer and a horseradish peroxidase reagent is made to the serum sample to measure Bf. The bilirubin bound to albumin is protected from oxidation. The rate of dissociation of the bound complexes must be significantly faster than the rate of bilirubin oxidation otherwise it would measure steady state Bf which is well below the equilibrium Bf. This underestimation of Bf can be resolved by measuring Bf at 2 or more peroxidase concentrations, typically using half the initial concentration of peroxidase (25,95). Then, both automated readouts of steady state Bf are used to derive the final equilibrium of Bf using a specific algorithm. The major limitation is that the sample dilution alters the intrinsic bilirubin binding properties (25,95). The apparent K for the albumin binding of bilirubin increases with sample dilution. The K increases significantly below albumin concentrations of about 0.6 g/dL most likely due to the dissociation of albumin oligomers that bind bilirubin less strongly than the albumin monomer. Thus, there is an apparent increase in available binding sites leading to the decrease in Bf. The sample dilution may also attenuate the effects of weak bilirubin binding competitors. Thus, it would be critical to use minimal sample dilution when evaluating bilirubin displacing effects of drugs. Despite these limitations, Bf measured using modified peroxidase method correlates directly and significantly with Bf measured at minimal sample dilution (25). Furthermore, numerous clinical studies in preterm and term infants have demonstrated that Bf measured using the modified peroxidase method is a more sensitive and specific predictor of neurotoxicity than TSB and/or the BAMR (Shown in Table 2). In Japan, the peroxidase method using a UB analyzer is routinely used to measure Bf for the management of unconjugated hyperbilirubinemia in preterm and term infants (96).
- Peroxidase method using zone fluidics. The Global FIA mini-Flo Pro also uses a horseradish peroxidase/glucose oxidase assay to measure serum Bf. Additionally, this method uses a minimally diluted sample compared to the UB analyzer (17). Signals measured by the spectrophotometer within the analyzer go directly into the Zone Fluidics software, which calculates the Bf concentration based on measurements at three different HRP/GO concentrations (97). “Zone stacks” are created for analyzing Bf concentration by aspirating an air bubble followed by 8 µL of sample, 4 µL of R3 (phosphate buffer with glucose and hydrogen peroxide), 4 µL of R2+R1 (HRP/GO enzyme and phosphate buffer, respectively), and lastly another air bubble; they are then transported to the spectrometer for analysis. The small sample dilution and processing of this technique contribute to a more precise measurement of Bf. Compared to the UB analyzer, this method is time consuming and often difficult because the enzyme dilution used depends on the expected Bf concentration of the sample. Other limitations include difficulty with standardization of the enzyme activity and stability of the prepared enzyme reagent. As with other binding assays, there is also possible interference with Bf measurement by conjugated bilirubin and photoisomers. However, this method is useful to study the bilirubin displacement effect of medications because of minimally diluted samples required to measure Bf (17). Van der Schoor et al. using zone fluidics reported that Bf levels vary considerably in preterm infants ≤32 weeks during the first postnatal week. They found that maximum Bf levels of 50 nmol/L occurred on day 4 and were higher in males (98). To date, there have been no BIND outcome studies reported using this instrumentation.
Fluorescent based methods
- Fluorometric method. A fluorometric assay utilizes fluorescently labeled fatty acid binding mutants (BL22) as probes that have a high specificity and high sensitivity for Bf. Acrylodan is the fluorophore that gives the probe its fluorescence. Bilirubin quenches acrylodan fluorescence by resonance energy transfer and the absorbance of bilirubin overlaps with the emission of acrylodan. The probe’s excitation wavelength is 375 nm and the emission wavelength of interest is 550 nm. This specifically chosen protein probe specifically binds to bilirubin and is insensitive to FFA and other metabolites found in serum (17). This method has been modified numerous times over the years. In the most updated version, Bf is measured using 5 µL of plasma in a disposable cartridge that contains the Bf sensor. The sensor comprises of a fatty acid binding protein labelled with a near infrared fluorophore that emits at 700 nm plus a second protein labeled with a fluorophore that emits at 820 nm. Upon binding Bf, the fluorescence at 700 nm is quenched. The degree of quenching of the 700 nm/820 nm ratios is used to calculate the Bf concentration. In a preliminary investigation in late preterm and term neonates during the first postnatal week, Bf measured using this method was found to have good correlation with the Bf measured by the UB analyzer using the peroxidase method (99). However, this method has not been compared with the modified peroxidase method in premature infants. Furthermore, there have been no BIND outcome studies reported using this instrumentation.
- Fluorescent protein-based detection. UnaG, a fluorescent protein from Japanese eel muscle that specifically binds to unconjugated bilirubin was studied by Iwatani et al. (100). This was the first large-scale clinical study demonstrating the applicability of this form of Bf measurement in newborn serum samples, requiring only a small volume of blood. UnaG-His-FLAG is generated by adding a polypeptide flag to the protein. A specific reaction mixture is made consisting of UnaG solution and diluted bilirubin solution to which the newborn serum or whole blood is added. The fluorescence intensity of the diluted sample is measured and Bf concentrations are determined from a standard calibration curve. The concentration of Bf measured by this method correlates strongly with those obtained by the conventional bilirubin oxidase method. Furthermore, performance of the UnaG method was unaffected by phototherapy and the presence of serum hemoglobin and lipid emulsion (100). This method has not yet been validated against the BIND outcome measures.
Potentiometry method
A novel method of Bf measurement in the serum was recently reported using electrolytic chemistry or potentiometry, which is primarily a measurement of electrical potential using an ion-specific electrode for chemical analysis (101). In this method, the potentiometric sensor has a polymeric ion-selective membrane and selectively measures “free ionic bilirubin” in the presence of other anions such as chloride, phosphate, pyruvate, lactate and others normally present in serum. The linear response range of the sensor (1.0 mM to 0.10 μM bilirubin, measured in a sodium phosphate buffer with pH 8.6) covers the clinically-relevant concentration of bilirubin in serum (5–500 μM). The components of the sensor were embedded in a paper-based device to provide a sensor that is disposable and suitable for POC. The method requires only 15 μL of sample and the analysis can be performed in 1 to 2 minutes. This method has not yet been studied in neonates (101).
Indirect method of free bilirubin measurement
Unconjugated Bf is essentially non-fluorescent in most solvents. The fluorescence of unconjugated Bf increases by binding to its native binding site on albumin. The fluorometric method that makes use of bound bilirubin’s natural fluorescence is being redeveloped for a POC system that uses <100 µL of whole blood and precludes the requirement for separation of serum or dilution of the specimen. The ‘B’ concentration {Eq. [1]} is close to TSB since the Bf concentration is much smaller until albumin binding is saturated. The reserve binding capacity (R) can be measured by adding excess bilirubin to the blood, resulting in an increase in fluorescence due to newly bound bilirubin. This method measures bilirubin binding capacity. The hematofluorometry results for bilirubin binding capacity and a calculated Bf are shown to be in good agreement with other more complex methods, including the peroxidase method. However, this POC method has not been validated in any clinical studies of BIND (17,32).
Summary
In conclusion, infants with excessive bilirubin production (indexed by TSB) or poor plasma bilirubin binding (indexed by Bf) have an increased risk of BIND. Both TSB and Bf measurement contribute critical information about the risk of BIND. Despite ample evidence for the usefulness of bilirubin binding measurement in assessing the risk for BIND, TSB remains the primary parameter used worldwide, except for Japan where both TSB and Bf are used successfully, to guide treatment with phototherapy and or exchange transfusion (27,96). The direct measurement of Bf by the modified peroxidase method provides the best risk assessment of BIND. Based on available methods, it is prudent to establish Bf population parameters at current threshold TSB levels routinely used to manage neonates with phototherapy and/or exchange transfusion for quantifying bilirubin binding in neonates. Such information will likely provide important additional information about the risk of BIND and need for intervention. Additionally, direct methods of Bf measurement using POC instruments are urgently needed for timely intervention and prevention of BIND in high-risk neonates. The newer POC instruments of Bf measurement and binding capacity appear promising, but will require to be standardized and validated for its usefulness in predicting BIND in premature and term neonates.
Acknowledgments
Funding: The work was supported by NIH R21 HD078744, NIH R21 DE021161 and NIH R03HD61084.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David K. Stevenson and Ronald J Wong) for the series “Neonatal Jaundice” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/pm-21-15
Peer Review File: Available at https://dx.doi.org/10.21037/pm-21-15.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pm-21-15). The series “Neonatal Jaundice” was commissioned by the editorial office without any funding or sponsorship. The evidence provided for bilirubin binding measurement was supported by NIH grants. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amin SB, Smith T, Timler G. Developmental influence of unconjugated hyperbilirubinemia and neurobehavioral disorders. Pediatr Res 2019;85:191-7. [Crossref] [PubMed]
- Amin SB. Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Semin Perinatol 2004;28:340-7. [Crossref] [PubMed]
- Scheidt PC, Graubard BI, Nelson KB, et al. Intelligence at six years in relation to neonatal bilirubin levels: follow-up of the National Institute of Child Health and Human Development Clinical Trial of Phototherapy. Pediatrics 1991;87:797-805. [PubMed]
- Oh W, Tyson JE, Fanaroff AA, et al. Association between peak serum bilirubin and neurodevelopmental outcomes in extremely low birth weight infants. Pediatrics 2003;112:773-9. [Crossref] [PubMed]
- Ip S, Chung M, Kulig J, et al. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics 2004;114:e130-53. [Crossref] [PubMed]
- Graziani LJ, Mitchell DG, Kornhauser M, et al. Neurodevelopment of preterm infants: neonatal neurosonographic and serum bilirubin studies. Pediatrics 1992;89:229-34. [PubMed]
- O'Shea TM, Dillard RG, Klinepeter KL, et al. Serum bilirubin levels, intracranial hemorrhage, and the risk of developmental problems in very low birth weight neonates. Pediatrics 1992;90:888-92. [PubMed]
- Newman TB, Klebanoff MA. Neonatal hyperbilirubinemia and long-term outcome: another look at the Collaborative Perinatal Project. Pediatrics 1993;92:651-7. [PubMed]
- Wennberg RP, Ahlfors CE, Bhutani VK, et al. Toward understanding kernicterus: a challenge to improve the management of jaundiced newborns. Pediatrics 2006;117:474-85. [Crossref] [PubMed]
- Amin SB. Bilirubin Binding Capacity in the Preterm Neonate. Clin Perinatol 2016;43:241-57. [Crossref] [PubMed]
- Pubchem.ncbi.nlm.nih.gov. 2021. Bilirubin(2-). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bilirubin_2
- Ruud Hansen TW. Phototherapy for neonatal jaundice--therapeutic effects on more than one level? Semin Perinatol 2010;34:231-4. [Crossref] [PubMed]
- Brodersen R. Binding of bilirubin to albumin. CRC Crit Rev Clin Lab Sci 1980;11:305-99. [Crossref] [PubMed]
- Ostrow JD, Mukerjee P, Tiribelli C. Structure and binding of unconjugated bilirubin: relevance for physiological and pathophysiological function. J Lipid Res 1994;35:1715-37. [Crossref] [PubMed]
- Calligaris SD, Bellarosa C, Giraudi P, et al. Cytotoxicity is predicted by unbound and not total bilirubin concentration. Pediatr Res 2007;62:576-80. [Crossref] [PubMed]
- Bratlid D. How bilirubin gets into the brain. Clin Perinatol 1990;17:449-65. [Crossref] [PubMed]
- Amin SB, Lamola AA. Newborn jaundice technologies: unbound bilirubin and bilirubin binding capacity in neonates. Semin Perinatol 2011;35:134-40. [Crossref] [PubMed]
- Jacobsen J. Binding of bilirubin to human serum albumin - determination of the dissociation constants. FEBS Lett 1969;5:112-4. [Crossref] [PubMed]
- Wells R, Hammond K, Lamola AA, et al. Relationships of bilirubin binding parameters. Clin Chem 1982;28:432-9. [Crossref] [PubMed]
- Jacobsen J. Studies of the affinity of human serum albumin for binding of bilirubin at different temperatures and ionic strength. Int J Pept Protein Res 1977;9:235-9. [Crossref] [PubMed]
- Roca L, Calligaris S, Wennberg RP, et al. Factors affecting the binding of bilirubin to serum albumins: validation and application of the peroxidase method. Pediatr Res 2006;60:724-8. [Crossref] [PubMed]
- Wennberg RP, Ahlfors CE, Rasmussen LF. The pathochemistry of kernicterus. Early Hum Dev 1979;3:353-72. [Crossref] [PubMed]
- Amin SB, Ahlfors CE. Bilirubin-binding capacity in premature infants. Pediatrics 2008;121:872-3; author reply 873. [Crossref] [PubMed]
- Koren R, Nissani E, Perlmutter-Hayman B. The kinetics of the reaction between bovine serum albumin and bilirubin. A second look. Biochim Biophys Acta 1982;703:42-8. [Crossref] [PubMed]
- Ahlfors CE, Wennberg RP, Ostrow JD, et al. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin Chem 2009;55:1288-99. [Crossref] [PubMed]
- Ahlfors CE. The Bilirubin Binding Panel: A Henderson-Hasselbalch Approach to Neonatal Hyperbilirubinemia. Pediatrics 2016;138:e20154378 [Crossref] [PubMed]
- Ahlfors CE, Bhutani VK, Wong RJ, et al. Bilirubin binding in jaundiced newborns: from bench to bedside? Pediatr Res 2018;84:494-8. [Crossref] [PubMed]
- Meisel P, Jährig D, Beyersdorff E, et al. Bilirubin binding and acid-base equilibrium in newborn infants with low birthweight. Acta Paediatr Scand 1988;77:496-501. [Crossref] [PubMed]
- Cashore WJ, Horwich A, Karotkin EH, et al. Influence of gestational age and clinical status on bilirubin-binding capacity in newborn infants. Sephadex G-25 gel filtration technique. Am J Dis Child 1977;131:898-901. [Crossref] [PubMed]
- Cashore WJ. Free bilirubin concentrations and bilirubin-binding affinity in term and preterm infants. J Pediatr 1980;96:521-7. [Crossref] [PubMed]
- Bender GJ, Cashore WJ, Oh W. Ontogeny of bilirubin-binding capacity and the effect of clinical status in premature infants born at less than 1300 grams. Pediatrics 2007;120:1067-73. [Crossref] [PubMed]
- Lamola AA, Bhutani VK, Du L, et al. Neonatal bilirubin binding capacity discerns risk of neurological dysfunction. Pediatr Res 2015;77:334-9. [Crossref] [PubMed]
- Amin SB, Wang H. Bilirubin Albumin Binding and Unbound Unconjugated Hyperbilirubinemia in Premature Infants. J Pediatr 2018;192:47-52. [Crossref] [PubMed]
- Kapitulnik J, Horner-Mibashan R, Blondheim SH, et al. Increase in bilirubin-binding affinity of serum with age of infant. J Pediatr 1975;86:442-5. [Crossref] [PubMed]
- Cashore WJ, Oh W, Brodersen R. Reserve albumin and bilirubin toxicity index in infant serum. Acta Paediatr Scand 1983;72:415-9. [Crossref] [PubMed]
- Kozuki K, Oh W, Widness J, et al. Increase in bilirubin binding to albumin with correction of neonatal acidosis. Acta Paediatr Scand 1979;68:213-7. [Crossref] [PubMed]
- Ebbesen F, Knudsen A. The risk of bilirubin encephalopathy, as estimated by plasma parameters, in neonates strongly suspected of having sepsis. Acta Paediatr 1993;82:26-9. [Crossref] [PubMed]
- Park W, Paust H, Schröder H. Lipid infusion in premature infants suffering from sepsis. JPEN J Parenter Enteral Nutr 1984;8:290-2. [Crossref] [PubMed]
- Ritter DA, Kenny JD, Norton HJ, et al. A prospective study of free bilirubin and other risk factors in the development of kernicterus in premature infants. Pediatrics 1982;69:260-6. [PubMed]
- Aranda JV, Varvarigou A, Beharry K, et al. Pharmacokinetics and protein binding of intravenous ibuprofen in the premature newborn infant. Acta Paediatr 1997;86:289-93. [Crossref] [PubMed]
- Amin SB, Miravalle N. Effect of ibuprofen on bilirubin-albumin binding affinity in premature infants. J Perinat Med 2011;39:55-8. [Crossref] [PubMed]
- Ambat MT, Ostrea EM Jr, Aranda JV. Effect of ibuprofen L-lysinate on bilirubin binding to albumin as measured by saturation index and horseradish peroxidase assays. J Perinatol 2008;28:287-90. [Crossref] [PubMed]
- Ahlfors CE. Effect of ibuprofen on bilirubin-albumin binding. J Pediatr 2004;144:386-8. [Crossref] [PubMed]
- Cooper-Peel C, Brodersen R, Robertson A. Does ibuprofen affect bilirubin-albumin binding in newborn infant serum? Pharmacol Toxicol 1996;79:297-9. [Crossref] [PubMed]
- Riccio J, Amin SB. Bilirubin displacing effect of Ibuprofen in premature infants with unconjugated hyperbilirubinemia. Abstract. E-PAS2012:752473.
- Thibaut C, Hazard A, Huon C, et al. Effect of ibuprofen on bilirubin-albumin binding during the treatment of patent ductus arteriosus in preterm infant. J Matern Fetal Neonatal Med 2011;24:7-9. [Crossref] [PubMed]
- Brodersen R, Robertson A. Ceftriaxone binding to human serum albumin: competition with bilirubin. Mol Pharmacol 1989;36:478-83. [PubMed]
- Fink S, Karp W, Robertson A. Ceftriaxone effect on bilirubin-albumin binding. Pediatrics 1987;80:873-5. [PubMed]
- Amin SB, Wang H. Orlando M. Intravenous ceftriaxone and bilirubin displacing effect in term neonates with unconjugated Hyperbilirubinemia. Abstract. E-PAS2018:4127.258.
- Amin SB, Harte T, Scholer L, et al. Intravenous lipid and bilirubin-albumin binding variables in premature infants. Pediatrics 2009;124:211-7. [Crossref] [PubMed]
- Amin SB. Effect of free fatty acids on bilirubin-albumin binding affinity and unbound bilirubin in premature infants. JPEN J Parenter Enteral Nutr 2010;34:414-20. [Crossref] [PubMed]
- Spear ML, Stahl GE, Paul MH, et al. The effect of 15-hour fat infusions of varying dosage on bilirubin binding to albumin. JPEN J Parenter Enteral Nutr 1985;9:144-7. [Crossref] [PubMed]
- Rubin M, Harell D, Naor N, et al. Lipid infusion with different triglyceride cores (long-chain vs medium-chain/long-chain triglycerides): effect on plasma lipids and bilirubin binding in premature infants. JPEN J Parenter Enteral Nutr 1991;15:642-6. [Crossref] [PubMed]
- Kim MH, Yoon JJ, Sher J, et al. Lack of predictive indices in kernicterus: a comparison of clinical and pathologic factors in infants with or without kernicterus. Pediatrics 1980;66:852-8. [PubMed]
- Amin SB, Ahlfors C, Orlando MS, et al. Bilirubin and serial auditory brainstem responses in premature infants. Pediatrics 2001;107:664-70. [Crossref] [PubMed]
- Hulzebos CV, Dijk PH, van Imhoff DE, et al. The bilirubin albumin ratio in the management of hyperbilirubinemia in preterm infants to improve neurodevelopmental outcome: a randomized controlled trial--BARTrial. PLoS One 2014;9:e99466 [Crossref] [PubMed]
- Govaert P, Lequin M, Swarte R, et al. Changes in globus pallidus with (pre)term kernicterus. Pediatrics 2003;112:1256-63. [Crossref] [PubMed]
- Amin SB, Saluja S, Saili A, et al. Auditory toxicity in late preterm and term neonates with severe jaundice. Dev Med Child Neurol 2017;59:297-303. [Crossref] [PubMed]
- Johnson L, Bhutani VK. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Perinatol 2011;35:101-13. [Crossref] [PubMed]
- Nakamura H, Yonetani M, Uetani Y, et al. Determination of serum unbound bilirubin for prediction of kernicterus in low birthweight infants. Acta Paediatr Jpn 1992;34:642-7. [Crossref] [PubMed]
- Ahlfors CE, Amin SB, Parker AE. Unbound bilirubin predicts abnormal automated auditory brainstem response in a diverse newborn population. J Perinatol 2009;29:305-9. [Crossref] [PubMed]
- Amin SB, Saluja S, Saili A, et al. Chronic Auditory Toxicity in Late Preterm and Term Infants With Significant Hyperbilirubinemia. Pediatrics 2017;140:e20164009 [Crossref] [PubMed]
- Amin SB, Wang H, Laroia N, et al. Unbound Bilirubin and Auditory Neuropathy Spectrum Disorder in Late Preterm and Term Infants with Severe Jaundice. J Pediatr 2016;173:84-9. [Crossref] [PubMed]
- Amin SB, Charafeddine L, Guillet R. Transient bilirubin encephalopathy and apnea of prematurity in 28 to 32 weeks gestational age infants. J Perinatol 2005;25:386-90. [Crossref] [PubMed]
- Amin SB, Wang H. Unbound unconjugated hyperbilirubinemia is associated with central apnea in premature infants. J Pediatr 2015;166:571-5. [Crossref] [PubMed]
- Oh W, Stevenson DK, Tyson JE, et al. Influence of clinical status on the association between plasma total and unbound bilirubin and death or adverse neurodevelopmental outcomes in extremely low birth weight infants. Acta Paediatr 2010;99:673-8. [Crossref] [PubMed]
- Taylor JA, Stout JW, de Greef L, et al. Use of a Smartphone App to Assess Neonatal Jaundice. Pediatrics 2017;140:e20170312 [Crossref] [PubMed]
- Doumas BT, Wu TW. The measurement of bilirubin fractions in serum. Crit Rev Clin Lab Sci 1991;28:415-45. [Crossref] [PubMed]
- Vreman HJ, Verter J, Oh W, et al. Interlaboratory variability of bilirubin measurements. Clin Chem 1996;42:869-73. [Crossref] [PubMed]
- van Imhoff DE, Dijk PH, Weykamp CW, et al. Measurements of neonatal bilirubin and albumin concentrations: a need for improvement and quality control. Eur J Pediatr 2011;170:977-82. [Crossref] [PubMed]
- Lo SF, Kytzia HJ, Schumann G, et al. Interlaboratory comparison of the Doumas bilirubin reference method. Clin Biochem 2009;42:1328-30. [Crossref] [PubMed]
- Conceição CM, Dornaus MF, Portella MA, et al. Influence of assessment site in measuring transcutaneous bilirubin. Einstein (Sao Paulo) 2014;12:11-5. [Crossref] [PubMed]
- Boo NY, Ishak S. Prediction of severe hyperbilirubinaemia using the Bilicheck transcutaneous bilirubinometer. J Paediatr Child Health 2007;43:297-302. [Crossref] [PubMed]
- Raimondi F, Lama S, Landolfo F, et al. Measuring transcutaneous bilirubin: a comparative analysis of three devices on a multiracial population. BMC Pediatr 2012;12:70. [Crossref] [PubMed]
- Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000;106:E17 [Crossref] [PubMed]
- Teran CG, Mohamed T, Casey J. Transcutaneous bilirubinometry: comparison of two multiwavelength devices in healthy term newborns. Eur J Pediatr 2011;170:1485-author reply 1487. [Crossref] [PubMed]
- Fouzas S, Karatza AA, Skylogianni E, et al. Transcutaneous bilirubin levels in late preterm neonates. J Pediatr 2010;157:762-6.e1. [Crossref] [PubMed]
- Maisels MJ, Ostrea EM Jr, Touch S, et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics 2004;113:1628-35. [Crossref] [PubMed]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297-316. [Crossref] [PubMed]
- Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics 2015;135:224-31. [Crossref] [PubMed]
- Engle WD, Jackson GL, Sendelbach D, et al. Assessment of a transcutaneous device in the evaluation of neonatal hyperbilirubinemia in a primarily Hispanic population. Pediatrics 2002;110:61-7. [Crossref] [PubMed]
- Nagar G, Vandermeer B, Campbell S, et al. Effect of Phototherapy on the Reliability of Transcutaneous Bilirubin Devices in Term and Near-Term Infants: A Systematic Review and Meta-Analysis. Neonatology 2016;109:203-12. [Crossref] [PubMed]
- Hassan Shabuj M, Hossain J, Dey S. Accuracy of transcutaneous bilirubinometry in the preterm infants: a comprehensive meta-analysis. J Matern Fetal Neonatal Med 2019;32:734-41. [Crossref] [PubMed]
- Hulzebos CV, Vader-van Imhoff DE, Bos AF, et al. Should transcutaneous bilirubin be measured in preterm infants receiving phototherapy? The relationship between transcutaneous and total serum bilirubin in preterm infants with and without phototherapy. PLoS One 2019;14:e0218131 [Crossref] [PubMed]
- Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics 2013;132:871-81. [Crossref] [PubMed]
- Schmidt ET, Wheeler CA, Jackson GL, et al. Evaluation of transcutaneous bilirubinometry in preterm neonates. J Perinatol 2009;29:564-9. [Crossref] [PubMed]
- Agrawal G, Garg K, Sitaraman S, et al. Comparison of Diagnostic Accuracy of Different Sites for Transcutaneous Bilirubin Measurement in Early Preterm Infants. Indian J Pediatr 2019;86:32-7. [Crossref] [PubMed]
- Karen T, Bucher HU, Fauchère JC. Comparison of a new transcutaneous bilirubinometer (Bilimed) with serum bilirubin measurements in preterm and full-term infants. BMC Pediatr 2009;9:70. [Crossref] [PubMed]
- Greco C, Iskander IF, El Houchi SZ, et al. Diagnostic Performance Analysis of the Point-of-Care Bilistick System in Identifying Severe Neonatal Hyperbilirubinemia by a Multi-Country Approach. EClinicalMedicine 2018;1:14-20. [Crossref] [PubMed]
- Boo NY, Chang YF, Leong YX, et al. The point-of-care Bilistick method has very short turn-around-time and high accuracy at lower cutoff levels to predict laboratory-measured TSB. Pediatr Res 2019;86:216-20. [Crossref] [PubMed]
- Kamineni B, Tanniru A, Vardhelli V, et al. Accuracy of Bilistick (a Point-of-Care Device) to Detect Neonatal Hyperbilirubinemia. J Trop Pediatr 2020;66:630-6. [Crossref] [PubMed]
- Munkholm SB, Krøgholt T, Ebbesen F, et al. The smartphone camera as a potential method for transcutaneous bilirubin measurement. PLoS One 2018;13:e0197938 [Crossref] [PubMed]
- Outlaw F, Nixon M, Odeyemi O, et al. Smartphone screening for neonatal jaundice via ambient-subtracted sclera chromaticity. PLoS One 2020;15:e0216970 [Crossref] [PubMed]
- Andreu Y, Ostra M, Ubide C, et al. Study of a fluorometric-enzymatic method for bilirubin based on chemically modified bilirubin-oxidase and multivariate calibration. Talanta 2002;57:343-53. [Crossref] [PubMed]
- Ahlfors CE. Measurement of plasma unbound unconjugated bilirubin. Anal Biochem 2000;279:130-5. [Crossref] [PubMed]
- Morioka I. Hyperbilirubinemia in preterm infants in Japan: New treatment criteria. Pediatr Int 2018;60:684-90. [Crossref] [PubMed]
- Ahlfors CE, Marshall GD, Wolcott DK, et al. Measurement of unbound bilirubin by the peroxidase test using Zone Fluidics. Clin Chim Acta 2006;365:78-85. [Crossref] [PubMed]
- van der Schoor LW, Dijk PH, Verkade HJ, et al. Unconjugated free bilirubin in preterm infants. Early Hum Dev 2017;106-107:25-32. [Crossref] [PubMed]
- Hegyi T, Chefitz D, Weller A, et al. Unbound bilirubin measurements in term and late-preterm infants. J Matern Fetal Neonatal Med 2020;1-7. [Crossref] [PubMed]
- Iwatani S, Nakamura H, Kurokawa D, et al. Fluorescent protein-based detection of unconjugated bilirubin in newborn serum. Sci Rep 2016;6:28489. [Crossref] [PubMed]
- Bell JG, Mousavi MPS, Abd El-Rahman MK, et al. Paper-based potentiometric sensing of free bilirubin in blood serum. Biosens Bioelectron 2019;126:115-21. [Crossref] [PubMed]
Cite this article as: Amin SB. Narrative review of bilirubin measurement and binding. Pediatr Med 2021;4:33.

