Pharmacotherapeutic options for children with COVID-19: a narrative review
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has broken out and spread quickly around the world, and soon become a global pandemic with daily increasing confirmed cases (1,2). World Health Organization (WHO) has announced it as a public health emergency of international concern (2). Until now, how to effectively prevent and treat COVID-19 is still the major problem human is facing. According to epidemiological studies all over the world, elderly patients and patients with underlying diseases, once get infected with SARS-CoV-2, are at high risk to have severe disease presentation and complications, and even die in the end (3,4). Fortunately, children and young adults with COVID-19 tend to have milder disease course and lower reported death rate (5,6).
Currently, supportive care is the primary way of treatment for COVID-19 (7). Many potential antiviral drugs have undergone clinical trials testing their efficacy and safety in treating COVID-19, the results of which, however, did not show significant effect in general except for remdesivir, and there are some controversies and uncertainties in the application of some drugs like hydroxychloroquine (HCQ) and chloroquine (CQ) (8-13). In addition, cytokine storm is one of the major causes of acute respiratory distress syndrome (ARDS) and multi-organ failure in critically ill patients (14). Immunomodulators, such as corticosteroids, immunoglobulin, interlukin-6 (IL-6) antagonists, etc., can effectively suppress the cytokine storm, and hence become an important part in the treatment to prevent disease progression in patients with COVID-19 (14). In children, it has been reported that COVID-19 is associated with a clinical hyperinflammatory syndrome resembling Kawasaki disease (KD), which was later named as multisystem inflammatory syndrome in children (MIS-C) (15-18). Therefore, pharmacological treatment, including intravenous immunoglobulin (IVIG), aspirin, corticosteroids, and anticoagulants may be applied in that case (18). Furthermore, antibacterial and antifungal drugs may be used as needed when patients have suspected or confirmed coinfection with pathogens other than SARS-CoV-2 (19).
The complexity of pharmacological treatment in children with COVID-19 spawned this review, intending to summarize reported pharmacotherapeutic options for children in literatures published to date, and follow up research findings regarding these options. We present the following article in accordance with the Narrative Review checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-20-103/rc).
Methods
Database and search strategy
We searched the database PubMed (pubmed.ncbi.nlm.nih.gov) with the searching terms and formula as follows: (COVID-19) AND ((children) OR (child) OR (pediatric)) AND ((pharmacotherapy) OR (“pharmacological treatment”) OR (medication) OR (drug) OR (treatment)). We included all types of clinical studies (clinical trials, observational studies, comparative studies) that reported pharmacological treatment data in pediatric patients with COVID-19 and published before November 5th, 2020. Case reports with pharmacotherapeutic regimen for children with COVID-19 were also included. Excluded articles or studies were (I) duplicate articles, (II) review articles, (III) articles not in English language, (IV) articles not available electronically, (V) study protocols, (VI) studies on SARS-CoV-2 detection/assay methods, (VII) studies on the influence of COVID-19 pandemic on health care practice for diseases other than COVID-19, (VIII) studies on psychological impact of COVID-19 pandemic, (IX) articles that did not include or report data from pediatric patients (≤18 years old) with COVID-19, (X) articles that reported suspected COVID-19 cases but had no evidence to confirm the diagnosis, (XI) articles that reported pregnant patients, (XII) articles that did not report pharmacological treatment for COVID-19 in children, (XIII) articles that did not report exact number of pediatric patients with COVID-19 that received pharmacological treatment, and (XIV) articles that did not report specific pharmacological treatment for COVID-19.
Data collection and analysis
Data of our interest included country (where the study was conducted or the patients were admitted and treated), the number of pediatric patients with COVID-19 reported in the article, the age and gender distributions of the patients, the number of pediatric patients presented with fever when diagnosed with COVID-19, the number of patients who died during the study or observation, the number of patients who needed ventilation or oxygen support [including nasal cannula or oxygen mask, high flow nasal cannula (HFNC), positive airway pressure, mechanical ventilation, extracorporeal membrane oxygenation (ECMO)], the number of patients with MIS-C, the medications used during the study or observation, and the number of patients used a particular medication. After collecting all the data needed, descriptive analyses were conducted to explore possible pharmacological treatment options for pediatric patients with COVID-19. The medications reported in this review are not necessarily effective in treating COVID-19.
Discussion
Publications included and distribution by country and age
With the search strategy, 5,496 publications were found in the search results. After screening with the inclusion and exclusion criteria, 119 publications were included in this review, of which 104 were case reports (please check the references of case reports at https://cdn.amegroups.cn/static/public/pm-20-103-1.pdf) and 15 were observational cohort studies (20-34). In total, 1,841 pediatric patients (1,024 males, 815 females, 2 without reported gender; 1,162 presented with fever; 25 died during the studying/observing period) with COVID-19 were reported in the 119 publications. Of the 1,841 patients, 154 were from case reports, 1,687 were from cohort studies. The characteristics of the patients from case reports are shown in Table 1.
Table 1
| Variables | Total | Oxygen/ventilation support | Mechanical ventilation | ECMO | MIS-C | Died |
|---|---|---|---|---|---|---|
| N | 154 | 82 | 32 | 6 | 36 | 8 |
| Male, n (%) | 91 (59.1) | 48 (58.5) | 19 (59.4) | 1 (16.7) | 20 (55.6) | 2 (25.0) |
| Age, mean (SD) | 7.1 (5.7) | 6.9 (6.1) | 7.3 (6.3) | 9.0 (4.1) | 9.0 (4.8) | 3.2 (4.3) |
| Coinfection, n (%) | 9 (5.8) | 3 (3.7) | 1 (3.1) | 0 (0) | 0 (0) | 2 (25.0) |
N, number of patients; ECMO, extracorporeal membrane oxygenation; MIS-C, multisystem inflammatory syndrome in children.
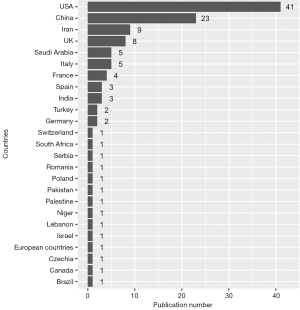
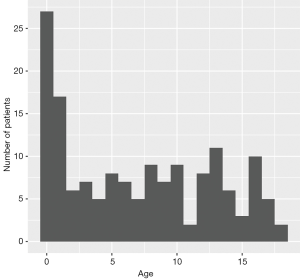
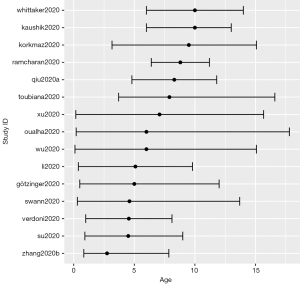
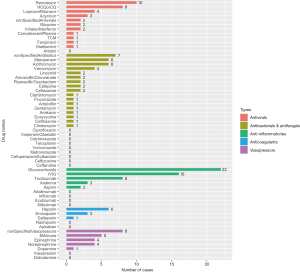
The distribution of all the publications by countries is shown in Figure 1. The United States of America had 41 studies or case reports published which was the most among the countries, and China ranked the second with 23 publications. The age distribution of case reports is shown in Figure 2, where most cases were less than 3 years old. The age distribution of cohort studies is shown in Figure 3.
Drugs used for pediatric patients with COVID-19
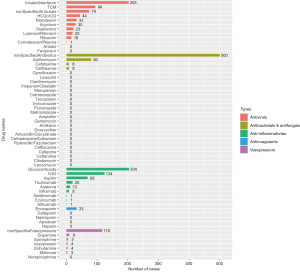
There are five main categories of drugs used in pediatric patients diagnosed with COVID-19: antivirals, antibacterials and antifungals, anti-inflammatories, anticoagulants, and vasopressors or inotropes. For patients complicated with autoimmune diseases or organ transplantation, immunosuppressants, such as tacrolimus, sirolimus, mycophenolate mofetil, etc. were co-administered along with other medications treating COVID-19. For patients complicated with leukemia, chemotherapeutic drugs like methotrexate, vincristine, daunorubicin were also applied. The number of patients who used the five main types of drugs from cohort studies and case reports are shown in Figure 4 and Figure 5, respectively.
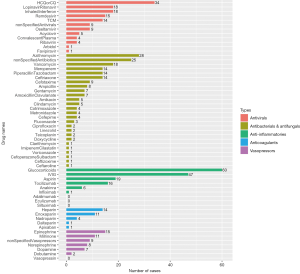
Drugs applied in COVID-19 children who needed oxygen or ventilation support
Among the 154 patients from case reports, 82 patients (53.2%) needed oxygen or ventilation support, 32 (20.8%) needed mechanical ventilation, and 6 (3.9%) were on ECMO. Specific drugs used in the 82 patients with oxygen or ventilation support are presented in Figure 6. HCQ or CQ was the most applied antiviral, with remdesivir ranking the second. Azithromycin was the most used antibacterial with 19 cases of application. Further, the drugs applied in the 32 children on mechanical ventilators had slightly different proportions, which are shown in Figure 7, with remdesivir being the most used antiviral instead of HCQ or CQ.
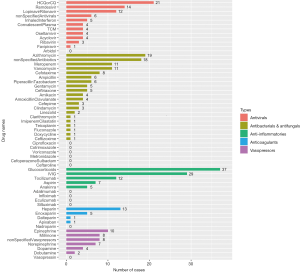
Antiviral drugs for COVID-19
Since the outbreak of COVID-19, great effort has been made to develop antiviral drugs with special efficacy of inhibiting the infection and proliferation of SARS-CoV-2. Potential antiviral drugs for COVID-19 including interferon, HCQ, CQ, remdesivir, lopinavir-ritonavir, ribavirin, arbidol, and favipiravir, were recommended in the beginning by the Diagnosis and Treatment Protocol for COVID-19 issued by the National Health Commission of the People’s Republic of China (35) as well as earlier published in vitro studies (36). Later, as the epidemic escalated into a pandemic, there is a surge of registered clinical trials testing the efficacy and safety of these drugs on patients with COVID-19, mainly on adult patients (37,38). Nevertheless, pediatricians are still facing a lack of evidence guiding the application of these drugs on pediatric patients, especially those of young age and physiologically immature. With the data from clinical studies in adult patients, efficacy of these antiviral drugs seems not definite.
Although HCQ and CQ are recommended against by authoritative COVID-19 treatment guidelines (7,39), they were granted Emergency Use Authorization (EUA) for treating COVID-19 by the US Food and Drug Administration (FDA) on March 28, 2020 (40), as initial clinical studies of HCQ and CQ in COVID-19 patients reported positive results on lowering viral load as well as reducing the severity of pneumonia (10,41). However, as more and more large-sample studies published since May, 2020, the attitude towards HCQ and CQ turned skeptical and even negative (8,12,42,43). Severe QT-prolongation adverse effect and increased death rate were reported in COVID-19 patients treated with HCQ or CQ (44). On June 5, the RECOVERY trial in UK stopped enrolling participants to the HCQ arm, considering there was no beneficial effect of HCQ, which was concluded with the unblinded data by chief investigators (45). On June 15, 2020, the US FDA revoked the EUA for CQ and HCQ based on several facts that earlier observational studies generated inconsistent evidence, a large randomized controlled trial showed no evidence of treatment benefit, and US treatment guidelines recommended against the use of CQ or HCQ in COVID-19 patients (46).
The original indications of CQ and HCQ for pediatric patients are chemoprophylaxis and treatment for malaria, juvenile rheumatoid arthritis (JRA), systemic lupus erythematosus (SLE), extraintestinal amebiasis, and liver abscess (47). For treating pediatric patients with COVID-19, investigated adult dosages were extrapolated to children through pharmacokinetic modeling and simulation. Based on the demographics of a white US population, Maharaj et al. (48) gave oral dosing algorithm of HCQ for pediatric patients. For Day 1, 6 mg/kg every 12 hours (q12h) (weight <50 kg); 400 mg q12h (weight ≥50 kg). For Day 2–5, 1.5 mg/kg q12h (weight <5 kg); 2.5 mg/kg q12h (weight 5 kg to <25 kg); 3 mg/kg q12h (weight 25 kg to <50 kg); 200 mg q12h (weight ≥50 kg). The dosing regimen given above is for experimental application of CQ and HCQ in pediatric patients. However, since CQ and HCQ are not recommended to be used in adults with COVID-19, they are unlikely to be applied as first-line treatment for children’s COVID-19 until further evidence confirms their efficacy.
In children, the most used antiviral drug is inhaled interferon (IFN). IFN is a group of low-molecular glycoproteins produced by host cells when the body is infected by virus (49). Type I IFNs-α/β have broad-spectrum antiviral activity, with both direct inhibitory effects on viral replication and supportive function to immune response of the host to clear virus infection. Zhou and colleagues (50) evaluated the effects of inhaled IFN-α2b on the clearance of SARS-CoV-2 in 77 adults, the results of which suggested that inhaled IFN-α2b could accelerate viral clearance and hasten the resolution of systemic inflammatory process by reducing interleukin-6 (IL-6), when compared to arbidol treatment alone. However, this is an exploratory study and the cohort size was small consisting only of moderate cases. The COVID-19 Treatment Guideline still recommends against the application of IFN on severe cases since no benefit was shown in patients with Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) (39). Given the inadequate evidence, the guideline recommends neither for nor against the use of IFN-β in treating mild or moderate COVID-19 cases (39).
Remdesivir is the only drug approved by the FDA for treating COVID-19. It is a phosphonamidate prodrug of an adenine nucleotide analogue with broad-spectrum antiviral activity against coronaviruses (51). Clinical data from adult patients showed inconsistent evidence on its efficacy (52-55). Studies have suggested that early application of remdesivir in patients with severe clinical manifestation of COVID-19 could have more evident benefit. This is consistent with the findings from the 104 case reports collected for this review that remdesivir is the most used antiviral in COVID-19 children on mechanical ventilator. The COVID-19 Treatment Guideline by National Institute of Health (NIH) has recommended the dosing regimens for both adult and pediatric patients (39). For hospitalized patients aged ≥12 years and weighing ≥40 kg, the recommendation is 200 mg intravenously infused (IV) over 30–120 minutes on Day 1 followed by 100 mg on Day 2 to Day 5 if the patients are not on mechanical ventilator, and the treatment duration expands to 10 days under the condition of mechanical ventilation. EUA for hospitalized pediatric patients with a weight of 3.5 kg to <40 kg includes remdesivir 5 mg/kg IV over 30–120 minutes on Day 1 followed by 2.5 mg/kg once daily since Day 2. The treatment duration is 5 or 10 days depending on the ventilation condition and clinical improvement. For children aged <12 years and weighing ≥40 kg, the dosing regimen is the same as that for adults. The common adverse effects of remdesivir include constipation, hypoalbuminemia, hypokalemia, anemia, thrombocytopenia, and increased total bilirubin (54).
Other antiviral drugs including lopinavir-ritonavir, ribavirin, favipiravir, arbidol, acyclovir, and oseltamivir had also been applied in COVID-19 children according to several case reports. However, up to now, there are no clear clinical evidence supporting the use of these antivirals for treating COVID-19 either in children or in adults (56). Except for remdesivir, the current COVID-19 Treatment Guideline by NIH recommends against the use of other antiviral drugs (39). Cautions should be exercised when experimentally applying these drugs on COVID-19 children in clinical settings.
In addition, convalescent plasma is also a potential therapeutic option for COVID-19. Five pediatric patients included in this review were reported to have received convalescent plasma for treatment (57). However, whether the clinical improvements observed in these patients were truly due to the treatment with convalescent plasma could not be answered by the case series. Unfortunately, data from a randomized controlled trial in adult patients with COVID-19 did not support the superior efficacy of convalescent plasma when added to standard treatment, compared to standard treatment (58). Despite the unfavorable results, limitations, such as small sample size, late treatment, short time frame of follow-up etc., call for further studies to provide stronger evidence on the use of COVID-19 convalescent plasma.
Other antimicrobial drugs
Co-infection was reported in patients with COVID-19, the prevalence of which could be as high as 50% among non-survivors according to previous studies (3). In this review, 9 out of 154 pediatric patients from the 104 case reports were confirmed to have coinfections, and 2 of them died during the treatment. Co-pathogens could be bacteria (Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, Chlamydia pneumonia, Mycoplasma pneumoniae), viruses (influenza, rhinovirus, enterovirus, respiratory syncytial virus), and fungi (Candida albicans, C. glabrata) (19,23,59). Therefore, in addition to anti-SARS-CoV-2 drugs, other antimicrobial drugs may be applied to patients with COVID-19 in clinical practice. From the data generated in this review, more than 500 pediatric patients had used antibiotics during the treatment. Most of the patients were put on broad-spectrum antibiotics empirically before microbiological testing, and later were re-evaluated to decide the discontinuation or not depending on the microbiological testing report. For example, in a prospective observational study by Toubiana et al. (20), 18 out of 21 children with COVID-19 received broad-spectrum antibiotics treatment for a median duration of 6.5 (range, 2–13) days, though all the testing results for bacteria afterwards were negative. Another observational cohort study conducted in UK reported 415 out 601 COVID-19 children used antibiotics during treatment, accounting for a large proportion of the patients (33). However, the NIH admits there are insufficient data to recommend empiric broad-spectrum antimicrobial therapy in patients with COVID-19 under critical care, and it also recommends that once the antimicrobials are initiated, their use should be reassessed daily to minimize the adverse effects (39). Given the concerns to the rising rate of antimicrobial resistance in general and the frequent application of antimicrobials in COVID-19 patients, antimicrobial stewardship program should be implemented appropriately to help with the rational use of this type of drugs (60).
Multisystem inflammatory syndrome in children
Since April 2020, some pediatric patients infected with SARS-CoV-2 had been reported to developed a severe illness, with symptoms such as fever, hypotension, severe abdominal pain, and myocardial dysfunction with elevation in cardiac damage markers (61). Additional laboratory features that were frequently reported in case series included lymphopenia, thrombocytopenia, and cytokine storm with elevated inflammatory biomarkers like IL-6 (20,27). This illness was named multisystem inflammatory syndrome in children (MIS-C) by the US Centers for Disease Control and Prevention (CDC) (16) and WHO (17). MIS-C resembles Kawasaki diseases in clinical presentations such as conjunctival injection, rash, and oral erythema. However, infected children diagnosed with MIS-C usually do not meet the diagnostic criteria of classic Kawasaki disease. Moreover, according to epidemiological data, the median age of cases with MIS-C was 9–10 years, which is consistent with the mean age (9 years) of children with MIS-C from the collected case reports, while Kawasaki disease predominantly occurs in children at 5 years of age or younger ((33,62,63).
Pharmacological treatment strategies for MIS-C
The treatment goals for MIS-C are to stabilize life-threatening manifestations and prevent long-term sequelae (64). Initiation of the treatment depends on the severity of the disease presentation. Under guidance from US CDC, treatment of MIS-C consists of supportive care and direct care against the inflammatory process (16). Inotropic support including inotropes or vasopressors should be applied properly when severe hypotension or cardiac dysfunction occurred. Anti-inflammatory treatment frequently involves IVIG and corticosteroids, but high-quality evidence on the efficacy of IVIG and corticosteroids used in MIS-C is currently not available. Other anti-inflammatory medications like biologics (tocilizumab, anakinra, infliximab, adalimumab, eculizumab, siltuximab, etc.) have also be reported in refractory case management. In addition, thromboprophylaxis with applications of anticoagulants (enoxaparin, heparin, nadroparin, dalteparin, apixaban, etc.) and aspirin is also part of the treatment protocol, considering the hypercoagulable state typically associated with MIS-C (16). Specific drugs used in COVID-19 children diagnosed with MIS-C from the collected case reports are shown in Figure 8.
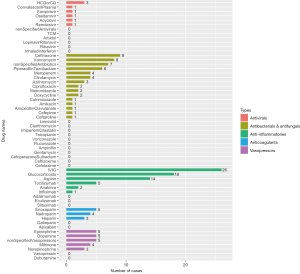
As for the right time to use immunomodulators, the American College of Rheumatology (ACR) recommends patients without life-threatening manifestations should get diagnostic evaluation for MIS-C and other possible etiologies before starting immunomodulatory treatment, while for the patients with life-threatening manifestations may start to use immunomodulators before the diagnostic evaluation is completed (64). A stepwise approach is recommended for the progression of immunomodulators with IVIG as the first tier and corticosteroids as adjunctive therapy in severe or refractory cases (64).
Biologics used in MIS-C
Anakinra is a recombinant human IL-1 receptor antagonist, commonly used to treat rheumatologic conditions (65), and reported to have efficacy in reducing mortality in septic patients with macrophage activation syndrome (MAS) (66). According to the clinical guidance for MIS-C by the ACR, it is of high consensus that anakinra treatment is safe in severe infections and in children with MIS-C (64). For children with MIS-C associated with SARS-CoV-2 infection, anakinra dosing >4 mg/kg/day intravenously or subcutaneously should be considered as immunomodulatory therapy, and it should be initiated before invasive mechanical ventilation (64).
Tocilizumab is IL-6 receptor antagonist, targeting both membrane-bound and soluble forms of receptors. Considering the elevated IL-6 level associated with negative outcomes in COVID-19 patients (3), neutralizing IL-6 with tocilizumab could be a potential treatment. However, evidence on the application of tocilizumab on COVID-19 is mixed, with some case series and a retrospective cohort study showing positive impact (67-69), but another cohort study concluding no effect on reducing intensive care unit (ICU) admission or mortality rate (70). In addition, high rate of bacterial and fungal infections associated with tocilizumab treatment had been noted in critically ill COVID-19 patients (71). Based on the available evidence, the ACR panel does not recommend the use of tocilizumab in most COVID-19 children with hyperinflammation (64).
Conclusions
As COVID-19 pandemic continues to spread across the world, treatment options gradually expand. For children, possible pharmacological treatment options could be generally divided into five categories: antivirals, antibacterials and antifungals, anti-inflammatories, anticoagulants and vasopressors. Inhaled interferon was the most used antiviral in cohort studies while hydroxychloroquine or chloroquine was the most in case reports, but remdesivir is the only antiviral approved for treating COVID-19. Glucocorticoids were the most used anti-inflammatories, with IVIG being the second. Different from adult patients, special considerations should be given to COVID-19 children meeting the diagnosis criteria of MIS-C. This review offers a comprehensive overview of the common medications used in clinical settings all over the world, but should be referred to with caution and flexibility depending on the actual condition of a specific patient.
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (No. 81874325), Key Innovative Team of Shanghai-Top-Level University Capacity Building in Clinical Pharmacy and Regulatory Science at Shanghai Medical College, Fudan University (HJW-R-2019-66-19), and Science and Technology Commission of Shanghai Municipality (No. 18DZ1910604, No.19DZ1910703 and No. 19XD1400900).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine for the series “Diagnosis and treatment of Covid-19 in children: experience from National Children’s Medical Center in China”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-20-103/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-20-103/coif). This series “Diagnosis and treatment of Covid-19 in children: experience from National Children’s Medical Center in China” was commissioned by the editorial office without any funding or sponsorship. GYH serves as an editor-in-chief of Pediatric Medicine and serves as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199-207. [Crossref] [PubMed]
- WHO. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. World Health Organization. 2020. Accessed 2020-11-07. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62. [Crossref] [PubMed]
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-42. [Crossref] [PubMed]
- Sinha IP, Harwood R, Semple MG, et al. COVID-19 infection in children. Lancet Respir Med 2020;8:446-7. [Crossref] [PubMed]
- Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med 2020;382:1663-5. [Crossref] [PubMed]
- WHO. Clinical management of COVID-19. World Health Organization: 2020.
- Rosenberg ES, Dufort EM, Udo T, et al. Association of Treatment With Hy-droxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 2020;323:2493-502. [Crossref] [PubMed]
- Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020;369:m1849. [Crossref] [PubMed]
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;56:105949. [Crossref] [PubMed]
- Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020;382:2411-8. [Crossref] [PubMed]
- Mahévas M, Tran VT, Roumier M, et al. Clinical efficacy of hydroxychloro-quine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 2020;369:m1844. Erratum in: BMJ 2020;369:m2328. [Crossref] [PubMed]
- Mehra MR, Desai SS, Ruschitzka F, et al. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multina-tional registry analysis. Lancet 2020. doi:
10.1016/S0140-6736(20)31180-6 . Re-traction in: Lancet 2020 Jun 5. Erratum in: Lancet 2020 May 30. PMID: 32450107.10.1016/S0140-6736(20)31180-6 - Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect 2020;80:607-13. [Crossref] [PubMed]
- Tam H, El Tal T, Go E, et al. Pediatric inflammatory multisystem syndrome temporally associated with COVID-19: a spectrum of diseases with many names. Cmaj 2020;192:E1093-6. [Crossref] [PubMed]
- CDC. Multisystem Inflammatory Syndrome (MIS-C). US Centers for Disease Control and Prevention. 2020. Accessed 2020-11-06. Available online: https://www.cdc.gov/mis-c/
- WHO. Multisystem inflammatory syndrome in children and adolescents with COVID-19. World Health Organization, 2020.
- Henderson LA, Canna SW, Friedman KG, et al. American College of Rheuma-tology Clinical Guidance for Multisystem Inflammatory Syndrome in Children As-sociated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Ver-sion 1. Arthritis Rheumatol 2020;72:1791-805. [Crossref] [PubMed]
- Lai CC, Wang CY, Hsueh PR. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect 2020;53:505-12. [Crossref] [PubMed]
- Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflamma-tory syndrome in children during the covid-19 pandemic in Paris, France: prospec-tive observational study. Bmj 2020;369:m2094. [Crossref] [PubMed]
- Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020;20:689-96. [Crossref] [PubMed]
- Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg Microbes Infect 2020;9:707-13. [Crossref] [PubMed]
- Wu Q, Xing Y, Shi L, et al. Coinfection and Other Clinical Characteristics of COVID-19 in Children. Pediatrics 2020;146:e20200961. [Crossref] [PubMed]
- Götzinger F, Santiago-Garcia B, Noguera-Julian A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020;4:653-61. [Crossref] [PubMed]
- Oualha M, Bendavid M, Berteloot L, et al. Severe and fatal forms of COVID-19 in children. Arch Pediatr 2020;27:235-8. [Crossref] [PubMed]
- Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020;26:502-5. [Crossref] [PubMed]
- Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771-8. [Crossref] [PubMed]
- Ramcharan T, Nolan O, Lai CY, et al. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): Cardiac Features, Management and Short-Term Outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol 2020;41:1391-401. [Crossref] [PubMed]
- Kaushik S, Aydin SI, Derespina KR, et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 In-fection (MIS-C): A Multi- institutional Study from New York City. J Pediatr 2020;224:24-9. [Crossref] [PubMed]
- Korkmaz MF, Ture E, Dorum BA, et al. The Epidemiological and Clinical Characteristics of 81 Children with COVID-19 in a Pandemic Hospital in Turkey: an Observational Cohort Study. J Korean Med Sci 2020;35:e236. [Crossref] [PubMed]
- Li H, Chen K, Liu M, et al. The profile of peripheral blood lymphocyte subsets and serum cytokines in children with 2019 novel coronavirus pneumonia. J Infect 2020;81:115-20. [Crossref] [PubMed]
- Zhang C, Gu J, Chen Q, et al. Clinical and epidemiological characteristics of pediatric SARS-CoV-2 infections in China: A multicenter case series. PLoS Med 2020;17:e1003130. [Crossref] [PubMed]
- Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. Bmj 2020;370:m3249. [Crossref] [PubMed]
- Whittaker E, Bamford A, Kenny J, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 2020;324:259-69. [Crossref] [PubMed]
- National Health Commission of People’s Republic of China. Diagnosis and Treatment Protocol for COVID-19 (Trial version 7). 2020.
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269-71. [Crossref] [PubMed]
- ClinicalTrials.gov [database on the Internet] 2020. Accessed: 2020-06-05. Available online: https://clinicaltrials.gov
- Chinese Clinical Trial Registry [database on the Internet] 2020. Accessed: 2020-06-05. Available online: http://www.chictr.org.cn/index.aspx.
- NIH. COVID-19 Treatment Guidelines. National Institute of Health. 2020. Accessed 2021-02-03. Available online: https://www.covid19treatmentguidelines.nih.gov/critical-care/pharmacologic-interventions/
- FDA. Chloroquine Phosphate and Hydroxychloroquine Sulfate EUA Letter of Authorization. US Food & Drug Administration, 2020.
- Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown ap-parent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Bioscience trends 2020;14:72-3. [Crossref] [PubMed]
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907-18. [Crossref] [PubMed]
- Boulware DR, Pullen MF, Bangdiwala AS, et al. A Randomized Trial of Hy-droxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med 2020;383:517-25. [Crossref] [PubMed]
- Jankelson L, Karam G, Becker ML, et al. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: A systematic review. Heart Rhythm 2020;17:1472-9. [Crossref] [PubMed]
- Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on hydroxychloroquine, 5 June 2020. 2020.
- FDA. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Au-thorization for Chloroquine and Hydroxychloroquine. US Food & Drug Admin-istration, 2020.
- Taketomo CK, Hodding JHH, Kraus DM. Pediatric & Neonatal Dosage Handbook. 25th ed. Wolters Kluwer, 2018.
- Maharaj AR, Wu H, Hornik CP, et al. Simulated Assessment of Pharmacoki-netically Guided Dosing for Investigational Treatments of Pediatric Patients With Coronavirus Disease 2019. JAMA Pediatr 2020;174:e202422. [Crossref] [PubMed]
- Shen KL, Yang YH. Diagnosis and treatment of 2019 novel coronavirus infec-tion in children: a pressing issue. World J Pediatr 2020;16:219-221. [Crossref] [PubMed]
- Zhou Q, Chen V, Shannon CP, et al. Interferon-alpha2b Treatment for COVID-19. Front Immunol 2020;11:1061. [Crossref] [PubMed]
- Martinez MA. Compounds with Therapeutic Potential against Novel Respira-tory 2019 Coronavirus. Antimicrob Agents Chemother 2020;64:e00399-20. [Crossref] [PubMed]
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020;383:1813-26. [Crossref] [PubMed]
- Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med 2020;383:1827-37. [Crossref] [PubMed]
- Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569-78. Erratum in: Lancet 2020;395:1694. [Crossref] [PubMed]
- Grein J, Ohmagari N, Shin D, et al. Compassionate Use of Remdesivir for Pa-tients with Severe Covid-19. N Engl J Med 2020;382:2327-36. [Crossref] [PubMed]
- Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospi-talized with Severe Covid-19. N Engl J Med 2020;382:1787-99. [Crossref] [PubMed]
- Schwartz SP, Thompson P, Smith M, et al. Convalescent Plasma Therapy in Four Critically Ill Pediatric Patients With Coronavirus Disease 2019: A Case Series. Crit Care Explor 2020;2:e0237. [Crossref] [PubMed]
- Li L, Zhang W, Hu Y, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 2020;324:460-70. [Crossref] [PubMed]
- Wehl G, Laible M, Rauchenzauner M. Co-infection of SARS CoV-2 and in-fluenza A in a Pediatric Patient in Germany. Klin Padiatr 2020;232:217-8. [Crossref] [PubMed]
- Huttner BD, Catho G, Pano-Pardo JR, et al. COVID-19: don't neglect antimi-crobial stewardship principles! Clin Microbiol Infect 2020;26:808-10. [Crossref] [PubMed]
- Rowley AH. Multisystem Inflammatory Syndrome in Children and Kawasaki Disease: Two Different Illnesses with Overlapping Clinical Features. J Pediatr 2020;224:129-32. [Crossref] [PubMed]
- Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem in-flammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020;142:429-36. [Crossref] [PubMed]
- Makino N, Nakamura Y, Yashiro M, et al. Descriptive epidemiology of Kawa-saki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol 2015;25:239-45. [Crossref] [PubMed]
- Henderson LA, Canna SW, Friedman KG, et al. American College of Rheuma-tology Clinical Guidance for Multisystem Inflammatory Syndrome in Children As-sociated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Ver-sion 2. Arthritis Rheumatol 2021;73:e13-29. [Crossref] [PubMed]
- Lopalco G, Rigante D, Giannini M, et al. Safety profile of anakinra in the management of rheumatologic, metabolic and autoinflammatory disorders. Clin Exp Rheumatol 2016;34:531-8. [PubMed]
- Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macro-phage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med 2016;44:275-81. [Crossref] [PubMed]
- Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol 2020;92:814-8. [Crossref] [PubMed]
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970-5. [Crossref] [PubMed]
- Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med 2020;76:31-5. [Crossref] [PubMed]
- Colaneri M, Bogliolo L, Valsecchi P, et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms 2020;8:695. [Crossref] [PubMed]
- Morena V, Milazzo L, Oreni L, et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med 2020;76:36-42. [Crossref] [PubMed]
Cite this article as: Ye Q, Wang G, Huang Y, Lu J, Zhang J, Zhu L, Zhu Y, Li X, Lan J, Li Z, Liu Y, Zhai X, Huang G, Li Z. Pharmacotherapeutic options for children with COVID-19: a narrative review. Pediatr Med 2021;4:26.