
A narrative review of delaying cord clamping 2020—who, what, when, where, why and how?
Introduction
Rationale/background
Until six decades ago, delayed cord clamping (DCC), on occasion with documented milking, was the rule, with several famous historical advocates including Erasmus Darwin and Aristotle in 300 B.C. The advent of medicalized delivery including third stage interventions to decrease post-partum hemorrhage (PPH) and Apgar scoring in infants affected adversely by maternal anesthesia led to increasingly rapid separation of the infant from placental circulation at birth. Early studies led to understanding of net placenta-infant transfusion at birth and residual placental volume thereafter (1-3).
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/pm-20-90).
Objective
This narrative review (4) describes recent lessons learned from term physiology and focuses on placental transfusion benefit and the time required for infants to achieve optimal cardiorespiratory transition.
Methods
Research selection
For this narrative review we used PubMed with a focus on human studies on DCC. A single animal study was included only to illuminate recent physiological understanding of umbilical cord milking (UCM) methodology, since human UCM clinical trials were not conducted using this technique inclusive of placental refill. Our search was not limited by publication year, language, or publication status.
Discussion/summary
Narrative
Weighing 26 term births with umbilical cord intact employing two methods showed a mean difference in weight of 87 g (95% CI: 64–110 g) to 116 g (95% CI: 72–160 g) with an estimated transfusion benefit of 83 mL (95% CI: 61–106 mL) to 110 mL (95% CI: 69–152 mL) respectively. This transfusion usually occurred by 2 minutes but sometimes continued for 5 minutes and contributed to 25–33% of total potential blood volume at birth in term infants (5). It is acknowledged this is a small, yet illuminating, sample size. It is known that fetoplacental blood flow increases exponentially from 19 weeks gestation to term (6) and that preterm infants have a greater proportion of fetoplacental blood volume in the placenta (up to 50% at 24 weeks gestation) thus standing to gain a greater percent of total potential blood volume at birth if allowed more time with umbilical cord intact while securing ventilation. A Doppler study of umbilical flow patterns after birth, and before cord clamping, in 30 vaginally delivered term infants with 1- and 5-minute Apgar scores of 9 and 10, was done. First breath occurred at median 8 s (IQR, 0–17 s) with median cord clamp time 5:36 (IQR, 03:57–09:02 min:sec). Venous flow was not demonstrated in 10% of infants but continued in 33% until the time of cord clamp (median 5:36 min:sec) and venous flow increased markedly with large breaths but stopped or reversed with crying. Arterial doppler flow was not demonstrated in 17% of infants and in another 40%, stopped at 4:22 (IQR, 2:29–7:17 min:sec) but palpable pulsations continued in 11 out of 12 of these infants. Pulsatile arterial flow was unidirectional toward the placenta or bidirectional to/from the placenta. These findings suggest that in spontaneously ventilating infants, umbilical flow continued for much longer than previously described and was unrelated to cessation of pulsation (7). A partially blinded randomized control trial (RCT) comparing 73 term fetuses randomized to DCC >5 minutes or ICC <20 s, who received cord clamping duration of 172±188 s vs. 28±76 s (P<0.002) respectively showed improved ferritin and brain myelination in areas important for early functional development at age 4 months (8). Mullen Scales of Early Learning testing were no different between groups but a positive correlation was noted between infant ferritin and brain myelin content (9).
A single-blinded RCT of healthy pregnant women (N=73) with term singleton fetuses who received either (ICC) (≤20 s) or DCC (≥ 5 minutes) had follow-up at 12 months and some had usable MRIs (n=41/58). At 12 months, infants with DCC had increased white matter brain growth in regions localized within the right and left internal capsules, the right parietal, occipital, and prefrontal cortex. Gender exerted no difference on any variables. The authors concluded 12-month-old infants with DCC had greater myelin content in important brain regions involved in motor function, visual/spatial, and sensory processing and that placental transfusion at birth appeared to increase myelin content in the early developing brain (9).
A recent review of neurodevelopmental outcomes in preterm infants (<33 weeks) examined DCC 30–60 s to ICC concluded that better neurodevelopment outcomes might occur among the smallest preterm infants (<30 weeks). However, all three available studies in this review, were underpowered to detect significant differences between groups. DCC appeared safe and was associated with similar subsequent neurodevelopmental outcomes when compared to ICC in preterm infants. Thus, they concluded that further studies are needed to confirm these findings and to assess the optimal timing of cord clamping (10).
A single center study compared 18–36 months long-term neurodevelopmental outcomes between two consecutive cohorts of extremely low birth weight (ELBW) infants with minimum DCC duration of 30 and 60 s, in a high-risk, low-income setting. ELBW infants born in the period 2009–2011 when the intended duration was >30 s DCC were compared to ELBW infants born in the period 2012–2015 when the intended duration was >60 s. Intent-to-treat analysis was performed between the two groups. A total of 84 of 98 ELBWs survived to discharge with one post-hospitalization death in each group. Of the 82 eligible ELBW survivors, 42 received Bayley III testing and >80% in both groups received the minimum intended duration of DCC. This study suggested longer DCC duration in ELBWs was associated with improved composite motor (but not language or cognitive) scores at 18–36 months (11).
A Swedish RCT of 400 term infants compared ICC and DCC (>180 s). At 4 months of age infants subjected to DCC had 45% higher mean ferritin concentration (117 vs. 81 µg/L, P<0.001) and a lower prevalence of iron deficiency [1 (0.6%) vs. 10 (5.7%), P=0.01, relative risk reduction 0.90; number needed to treat =20]. There were no significant differences between groups in postnatal respiratory symptoms, polycythemia, or hyperbilirubinemia requiring phototherapy. DCC, compared to ICC, showed improved iron status and reduced prevalence of iron deficiency at 4 months of age and reduced prevalence of neonatal anemia. DCC seems to benefit full term infants even in regions with a relatively low prevalence of iron deficiency anaemia (12).
A RCT of 540 late preterm and term infants born vaginally in Kathmandu, Nepal, in 2014 compared ICC ≤60 s to DCC >180 s. Main outcomes included hemoglobin and anemia levels at 8 months of age with secondary outcomes being prevalence of anemia at 12 months of age. DCC reduced anemia at 8 and 12 months of age in a high-risk population, which may have major positive effects on infants’ health and development (13). Iron metabolism in the brain is complex and vital to optimal myelination (14).
The safety, feasibility and efficacy of DCC (30–45 s) compared to ICC (5–10 s) among infants 22–27 weeks gestation concluded that among infants born at an average GA of 24 weeks’, DCC appeared safe, logistically feasible, and offers hematological and circulatory advantages compared with ICC. Forty mother-infant pairs randomized in the first 24 h of life showed blood pressures were lower in the ICC group than in the DCC group, despite a threefold greater incidence of treatment for hypotension (45% vs. 12%, P<0.01). This early (first 24 hours of life) hemodynamic stability is desirable in the very preterm infant (15).
A recent systematic review shows there is no clinically relevant difference in cord blood gases between delaying cord clamping for 1 or 2 minutes (16). Longer durations and relationship to cord gases are unstudied.
Optimal duration of DCC
The optimal duration of DCC is unknown. It is known that elective CS at term can occur without increased risk of excessive maternal blood loss: A pilot observational study compared ICC (n=112) and 120 s DCC (n=39) with regard to maternal blood loss from two periods, 2012–2013 and 2013-2014, respectively. Mean estimated blood loss in the DCC pilot group was 174 mL less compared to ICC. Maternal transfusion risk was also decreased from 18.8% ICC to 2.7% DCC (17).
In term infants, prior meta-analysis of DCC, defined as >2 minutes, found advantageous hematological indexes, reduced anemia and clinically insignificant increased polycythemia risk (18). In preterm infants, earlier meta analyses of DCC, defined as >20–30 s, had established decreased incidence of intraventricular hemorrhage (IVH), decreased blood transfusion risk, and increased survival (19-21).
A systematic review and meta-analysis including 18 RCTs compared delayed vs. early clamping in 2,834 infants <37 weeks gestation. Most infants were allocated to DCC ≥60 s. DCC reduced hospital mortality (risk ratio, 0.68; 95% CI: 0.52 to 0.90; risk difference, −0.03; 95% confidence interval, −0.05 to −0.01; P=0.005; number needed to benefit, 33; 95% CI: 20–100; high GRADE, with I2=0, i.e., no heterogeneity) including 3 trials with 996 infants ≤28 weeks’ gestation, DCC reduced hospital mortality (RR, 0.70; 95% CI: 0.51 to 0.95; risk difference, −0.05; 95% CI: −0.09 to −0.01; P=0.02, NNB =20; 95% CI: 11–100; I2=0). Subgroup analyses showed DCC decreased incidence of low Apgar score at 1 minute, but not at 5 minutes, and did not reduce the incidence of intubation for resuscitation (22). In contrast, Song et al. found in a single center prospective observation study of 353 consecutive infants <33 weeks gestation had a reduced delivery room (DR) intubation risk, 22% vs. 11% (P=0.004) in infants who received 30–45 s compared to subsequent infants receiving 60–75 s DCC, respectively. This included 96 infants <29 weeks gestation who had reduced DR intubation risk, 55% vs. 33% (P=0.03) in infants who received 30–45 vs. 60–75 s DCC, respectively (23). These infants also had diminished any-intubation risk during hospitalization. Infants <33 weeks gestation had a reduced any-intubation risk, 40% vs. 27% (P=0.007) in infants who received 30–45 vs. 60–75 s DCC, respectively. Infants <29 weeks gestation (n=96) who received 30–45 vs. 60–75 s DCC had reduced any-intubation risk of 89% vs. 63% (P=0.003), respectively. Consecutive inborn very preterm infants (n=640, 83% of whom received DCC) from this same center have shown further reductions in DR intubation and any-intubation risk as DCC duration has been prolonged to 2–3 minutes (24). These findings are now also noted in a multi-center retrospective study from the NICHD with 3,116 infants in 2016–2017 (40% of whom were exposed to placental transfusion) where mortality was reduced at 36 weeks PMA in the exposed group compared to the majority without this exposure (25). Interestingly, risk of DR positive pressure ventilation (PPV) and intubation were decreased from 87.9% to 80.6% and 65.7% to 58.3% respectively (both differences with P<0.0001). While the nature of these retrospective data does not exclude selection bias, they are reassuring, in that DCC when feasible, may provide improved survival to discharge and other short-term outcome advantages to the smallest of our infants.
Rabe’s meta-analysis from 40 studies involved 4,884 infants and their mothers, mostly from high-income nations with infants 24–36 weeks gestation and inclusive of multiple births. While the grade of evidence was mostly low due to imprecision and unclear risk of bias, DCC was associated with reduction in infants dying before discharge compared with ECC [average risk ratio (aRR) 0.73, 95% CI: 0.54 to 0.98]. No studies reported on ‘Death or neuro-developmental impairment’ in the early years. There was insufficient evidence to showing what DCC duration was best, one or several minutes. They conclude that, “whilst the current evidence supports not clamping the cord before 30 s at preterm births, future trials could compare different lengths of delay.” (19).
Many centers have embraced prolonged DCC to 2–3 minutes in preterm infants, including the smallest, as these infants have the most to benefit in terms of residual placental volume and may require more time to establish cardiopulmonary and hemodynamic transition (26,27). See Figure 1.
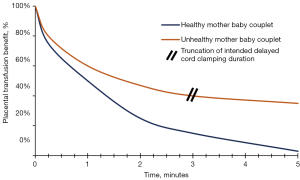
Absolute contraindications for DCC include perinatal sentinel events such as premature placental separation, cord prolapse, uterine rupture and certain maternal life-threatening events. Relative contraindications include prolonged infant apnea. Truncation of intended DCC duration is appropriate if infant cardiac output and/or utero-placental integrity are not reassuring (28).
Adverse maternal effects PPH
Early cord clamping was included in a bundle to prevent PPH in the last century, however it is not clear if the duration of cord clamping (immediate vs. few minutes) after birth has an impact on PPH. A Cochrane meta-analysis of term, singleton deliveries showed that DCC does not increase the risk of any PPH (>500 mL) (RR 1.17, 95% CI: 0.94–1.44, 5 trials, n=2,260 mothers), severe PPH (>1,000 mL) (RR 1.04, 95% CI: 0.65–1.6, 5 trials, n=2,066 mothers), mean blood loss [mean difference (MD) 5.11 mL; 95% CI: −23.18, 33.39; 2 trails, n=1,345 mothers], or decrease in maternal hemoglobin 24−72 h post-partum (MD −0.12 g/dL; 95% CI: −0.30 to 0.06; 3 trials, n=1,128 mothers) (29). The duration of DCC in the studies included in this meta-analysis varied from 1 minute, 10 s after effective breathing (mean 94 s), 2 minutes, 3 minutes, 5 minutes or until cord stopped pulsating, or after placental descent in the vagina; only 1 study included cesarean section (CS) deliveries (30). Risk of any (>500 mL) or major (>1,000 mL) maternal PPH was not increased (17,29,31-37).
Previously, the majority of the RCTs excluded CS, or the few that did include CS did not report maternal blood loss. Recent RCTs including CS did not show an increase in blood loss with at least 1 minute of DCC (31,33). A pilot study including 37 mothers who underwent CS showed that there is no increase in excessive blood loss with 120 s DCC (17). Multiple gestation is another sub population excluded from the majority of studies. Two retrospective cohort studies of multiples including a total of 513 mothers showed DCC >30 s did not increase the risk of maternal bleeding or PPH (32,38). One large RCT that studied at least 60 s DCC in preterm births <30 weeks gestation included both CS and multiple gestation showed no increase in maternal transfusion for blood loss (39).
Different methods have been used to quantify blood loss in these studies. Weighing sponges to get an accurate quantitative measurement of blood loss is recommended compared to visual assessment which overestimates blood loss. However, a recent Cochrane review, based on two RCTs of vaginal deliveries, showed no difference between calibrated drapes method vs. visual estimation or the calibrated drapes vs. gravimetric method (40). Another standard for measuring blood loss involves extraction of hemoglobin from soiled sponges and measurement of the hemoglobin level in the suction canister fluid to calculate the volume using the baseline maternal hemoglobin value.
A study of 50 CS patients showed estimation of mean blood loss using visual assessment (928 mL) and quantitative gravimetric method weighing soiled sponges and measuring the fluid in the suction canisters (822 mL) overestimated the blood loss significantly compared to the reference standard of extraction assay (470 mL). This study showed that a novel colorimetric image analysis method using a mobile application for scanning the soiled sponges and suction canister fluid (572 mL) had the best accuracy and correlation to the reference standard (41). This study highlights the significant limitation of the accuracy, and overestimation of blood loss during deliveries with current methodology. It is important that reliable methods to quantify blood like calorimetric image analysis are implemented in clinical care and in future research studies to evaluate the risk of PPH in longer duration of DCC. Measuring blood loss using colorimetric image analysis is superior to currently and previously employed visual estimation or gravimetric methods.
UCM vs. DCC
A detailed review of UCM is beyond the scope of this review but it may have utility in exceptional cases where prolonging duration may be considered unsafe. UCM may occur before or after cord clamping. The hemodynamic effects of cord milking in ventilated sick preterm sheep have shown clearly the need for a standardized UCM technique with placental refill to achieve effective placental transfusion of blood while not disrupting cerebral circulatory autoregulation (42). Examples of UCM technique heterogeneity include: stripping before/after cord clamping, number of times the cord is stripped, length of the cord that is stripped, stripping speed, and alternating cord pinching to allow placental refill. We limit his discussion to relevant technical aspects and studied comparison to DCC.
Term infants
An Indian single center RCT compared UCM (n=100) vs. DCC (90 s, n=100) in term infants (43-45). Both Jaiswal et al. studies and Agarwal et al. utilized the same study cohort. Jaiswal et al. reported no difference between UCM and DCC in hemoglobin (30 minutes, 48 hours, 6 weeks), packed cell volume (30 minutes, 48 hours), serum bilirubin (48 hours), or ferritin (6 weeks) (45). Jaiswal et al. also reported no differences between UCM and DCC in cerebral blood flow velocity and doppler indices in middle cerebral artery, as well as other hemodynamic outcomes (mean blood pressure, heart rate, and respiratory rate) up to 48 hours (44). Agarwal et al. performed a 12-month follow-up on 81% (161/200) of the original cohort (43). Their study found no significant differences in ferritin and hemoglobin levels as well as in growth parameters in infants at one year of age.
Yadav et al., another Indian single center RCT (same center and shared authors as Jaiswal et al. but used a new patient cohort) compared UCM (n=100) vs. DCC (90 s, n=100) in term infants (46). The same milking technique as Jaiswal et al. was used by Yadav et al. except they cord clamped at <10 s and they only stripped the cord twice. Contrary to Jaiswal et al.’s findings, Yadav et al. reported the DCC group had higher ferritin at 6 weeks of life compared the UCM group (44,46). Similar to Jaiswal et al., they reported no difference between UCM and DCC in hemoglobin (30 minutes, 48 hours, 6 weeks), hematocrit (30 minutes, 48 hours), or serum bilirubin (48 hours).
A Thai single center RCT compared neonatal and maternal outcomes in term infants who received either UCM (n=84) or DCC (n=84) (47). Their study reported no difference in infant hematocrit (6–24 hours, 48–72 hours), hemoglobin (6–24 hours, 48–72 hours) total bilirubin (48–72 hours), jaundice, phototherapy, length of stay, or maternal postpartum blood loss.
A Turkish single center RCT compared the thiol-disulfide balance in term infants (n=112) that received either DCC (90–120 s) or UCM (thrice, stripped 20 cm for 2 s) (48). The authors state lower thiol levels are an indicator of higher oxidative stress. Statistical differences directly between the DCC and UCM groups were not reported but could be conservatively estimated using the provided mean, standard deviation, and sample size coupled with a Bonferroni correction and assuming unequal variance. Using this method, they demonstrated UCM had statistically higher total thiol, disulfide, disulfide/native thiol, disulfide/total thiol, and lower native thiol/total thiol levels. There was no difference in native thiol.
Preterm infants
Barboza et al., a systematic review and meta-analysis, performed a subanalysis of five RCTs that compared DCC vs. UCM in preterm infants (49-54). Compared to DCC, UCM did not reduce mortality, IVH, patent ductus arteriosis, or need of blood transfusion but increased mean blood pressure (mean difference 3.7, 95% CI: 0.6 to 6.9) and Hb (mean difference 0.3, 95% CI: 0.2 to 0.8).
A multicenter RCT reported infants who received UCM (n=70) had higher language and cognitive scores at 22 to 26 months of corrected age compared to those who received DCC (n=65). There was no difference in rates of mild or moderate to severe neurodevelopmental impairment (55).
A retrospective cohort study compared DCC, UCM, and DCC + UCM (56). Their findings are difficult to clinically evaluate due to the presence of confounding by indication, as mentioned in their limitations.
One RCT of UCM compared to DCC in preterm infants was suspended for danger of increased risk of severe IVH with UCM in the least mature infants (50,57). It is possible that use of standardized UCM technique with placental refill may have had different results.
Upcoming studies
A Saudi Arabian RCT comparing DCC vs. UCM has recently completed recruiting preterm infants in order to primarily evaluate their hematological parameters (58). There are also two ongoing RCTs still recruiting patients to compare UCM vs. DCC in preterm infants. First is a single center Saudi Arabian study primarily evaluating IVH (59). Second is a single center Turkish study intending to primarily evaluate stem cell concentrations (60). Both of these studies also intend to report several other neonatal and maternal secondary outcomes.
Summary
The overwhelming preponderance of available evidence suggests that DCC for 2–5 minutes is employable in the majority of deliveries. In ~10% of births, it may not be practicable, or the intended duration may need to be truncated for reasons of perceived danger to maternal or infant health outcomes. Maternal blood loss using quantitative techniques presents opportunity for future study and is particularly important for multiple gestation and in operative deliveries. Future studies of UCM need employ standard techniques including UCM with placental refill. Long-term studies particularly focused on brain myelination and neurodevelopment outcomes are critical to ensure long term viability of optimal DCC duration as standard of care for ~90% of live births.
Acknowledgments
First Five of Santa Clara County, Valley Medical Center Foundation, County of Santa Clara Health and Human Services, Marshall Pediatrics, Marshall University Joan C. Edwards School of Medicine.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine. The article was sent for external peer review organized by the editorial office.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/pm-20-90
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-90). The authors have no conflict of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yao AC, Lind J. Placental transfusion. Am J Dis Child 1974;127:128-41. [PubMed]
- Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet 1969;2:871-3. [Crossref] [PubMed]
- Yao AC, Wist A, Lind J. The blood volume of the newborn infant delivered by caesarean section. Acta Paediatr Scand 1967;56:585-92. [Crossref] [PubMed]
- Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med 2006;5:101-17. [Crossref] [PubMed]
- Farrar D, Airey R, Law GR, et al. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG 2011;118:70-5. [Crossref] [PubMed]
- Sutton MS, Theard MA, Bhatia SJ, et al. Changes in placental blood flow in the normal human fetus with gestational age. Pediatr Res 1990;28:383-7. [Crossref] [PubMed]
- Boere I, Roest AA, Wallace E, et al. Umbilical blood flow patterns directly after birth before delayed cord clamping. Arch Dis Child Fetal Neonatal Ed 2015;100:F121-5. [Crossref] [PubMed]
- Mercer JS, Erickson-Owens DA, Deoni SCL, et al. Effects of Delayed Cord Clamping on 4-Month Ferritin Levels, Brain Myelin Content, and Neurodevelopment: A Randomized Controlled Trial. J Pediatr 2018;203:266-72 e2.
- Mercer JS, Erickson-Owens DA, Deoni SCL, et al. The Effects of Delayed Cord Clamping on 12-Month Brain Myelin Content and Neurodevelopment: A Randomized Controlled Trial. Am J Perinatol 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Nobile S, Tenace MA. Long-Term Neurodevelopmental Outcomes of Placental Transfusion (Delayed Cord Clamping or Cord Milking) Compared to Immediate Cord Clamping in Preterm Infants. EC Pulmonology and Respiratory Medicine 2019:552-5.
- Narasimhan SR, Govindaswami B, Vallejo M, et al. Long-term neurodevelopment outcomes at 18 to 36 months of life in extremely low birth weight infants who received delayed cord clamping for a minimum of 30 and 60 seconds. Pediatrics 2019;144:604.
- Andersson O, Hellstrom-Westas L, Andersson D, et al. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ 2011;343:d7157. [Crossref] [PubMed]
- Kc A, Rana N, Malqvist M, et al. Effects of Delayed Umbilical Cord Clamping vs. Early Clamping on Anemia in Infants at 8 and 12 Months: A Randomized Clinical Trial. JAMA Pediatr 2017;171:264-70. [Crossref] [PubMed]
- Moller HE, Bossoni L, Connor JR, et al. Iron, Myelin, and the Brain: Neuroimaging Meets Neurobiology. Trends Neurosci 2019;42:384-401. [Crossref] [PubMed]
- Backes CH, Huang H, Iams JD, et al. Timing of umbilical cord clamping among infants born at 22 through 27 weeks’ gestation. J Perinatol 2016;36:35-40. [Crossref] [PubMed]
- Nudelman MJR, Belogolovsky E, Jegatheesan P, et al. Effect of Delayed Cord Clamping on Umbilical Blood Gas Values in Term Newborns: A Systematic Review. Obstet Gynecol 2020;135:576-82. [Crossref] [PubMed]
- Chantry CJ, Blanton A, Tache V, et al. Delayed cord clamping during elective cesarean deliveries: results of a pilot safety trial. Matern Health Neonatol Perinatol 2018;4:16. [Crossref] [PubMed]
- Hutton EK, Hassan ES. Late vs. early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA 2007;297:1241-52. [Crossref] [PubMed]
- Rabe H, Gyte GM, Diaz-Rossello JL, et al. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2019;9:CD003248 [Crossref] [PubMed]
- Backes CH, Rivera BK, Haque U, et al. Placental transfusion strategies in very preterm neonates: a systematic review and meta-analysis. Obstet Gynecol 2014;124:47-56. [Crossref] [PubMed]
- Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst Rev 2004;CD003248 [Crossref] [PubMed]
- Fogarty M, Osborn DA, Askie L, et al. Delayed vs. early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol 2018;218:1-18. [Crossref] [PubMed]
- Song D, Jegatheesan P, DeSandre G, et al. Duration of Cord Clamping and Neonatal Outcomes in Very Preterm Infants. PLoS One 2015;10:e0138829 [Crossref] [PubMed]
- Govindaswami B, Nudelman M, Narasimhan SR, et al. Eliminating Risk of Intubation in Very Preterm Infants with Noninvasive Cardiorespiratory Support in the Delivery Room and Neonatal Intensive Care Unit. Biomed Res Int 2019;2019:5984305 [Crossref] [PubMed]
- Kumbhat N, Eggleston B, Davis AS, et al. Placental transfusion and short-term outcomes among extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2020:fetalneonatal-2019-318710.
- Haas J, Dillon B, Lewis K, et al. Integrating regionalized high-risk perinatal care. Submitted to Vermont Oxford Network 2020.
- Song D, Jegatheesan P, Huang A, et al. Delayed cord clamping for 2-3 minutes further reduces RBC transfusion in very preterm infants. Pediatric Academic Societies 2020.
- Committee on Obstetric P. Committee Opinion No. 684: Delayed Umbilical Cord Clamping After Birth. Obstet Gynecol 2017;129:e5-10. [PubMed]
- McDonald SJ, Middleton P, Dowswell T, et al. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev 2013;CD004074 [Crossref] [PubMed]
- Ceriani Cernadas JM, Carroli G, Pellegrini L, et al. The effect of early and delayed umbilical cord clamping on ferritin levels in term infants at six months of life: a randomized, controlled trial. Arch Argent Pediatr 2010;108:201-8. [PubMed]
- Purisch SE, Ananth CV, Arditi B, et al. Effect of Delayed vs. Immediate Umbilical Cord Clamping on Maternal Blood Loss in Term Cesarean Delivery: A Randomized Clinical Trial. JAMA 2019;322:1869-76. [Crossref] [PubMed]
- Ruangkit C, Leon M, Hassen K, et al. Maternal bleeding complications following early versus delayed umbilical cord clamping in multiple pregnancies. BMC Pregnancy Childbirth 2018;18:131. [Crossref] [PubMed]
- Withanathantrige M, Goonewardene I. Effects of early versus delayed umbilical cord clamping during antepartum lower segment caesarean section on placental delivery and postoperative haemorrhage: a randomised controlled trial. Ceylon Med J 2017;62:5-11. [Crossref] [PubMed]
- Chien PC, Yang CC, Gau ML, et al. The Impact of Late Umbilical Cord Clamping on Neonatal Jaundice and Postpartum Hemorrhage: A Randomized Controlled Trail. Hu Li Za Zhi 2015;62:41-53. [PubMed]
- De Paco C, Herrera J, Garcia C, et al. Effects of delayed cord clamping on the third stage of labour, maternal haematological parameters and acid-base status in fetuses at term. Eur J Obstet Gynecol Reprod Biol 2016;207:153-6. [Crossref] [PubMed]
- Andersson O, Domellof M, Andersson D, et al. Effects of delayed cord clamping on neurodevelopment and infection at four months of age: a randomised trial. Acta Paediatr 2013;102:525-31. [Crossref] [PubMed]
- Lalonde A, Daviss BA, Acosta A, et al. Postpartum hemorrhage today: ICM/FIGO initiative 2004-2006. Int J Gynaecol Obstet 2006;94:243-53. [Crossref] [PubMed]
- Jegatheesan P, Belogolovsky E, Nudelman M, et al. Neonatal outcomes in preterm multiples receiving delayed cord clamping. Arch Dis Child Fetal Neonatal Ed 2019;104:F575-81. [Crossref] [PubMed]
- Tarnow-Mordi W, Morris J, Kirby A, et al. Delayed versus Immediate Cord Clamping in Preterm Infants. N Engl J Med 2017;377:2445-55. [Crossref] [PubMed]
- Diaz V, Abalos E, Carroli G. Methods for blood loss estimation after vaginal birth. Cochrane Database Syst Rev 2018;9:CD010980 [Crossref] [PubMed]
- Doctorvaladan SV, Jelks AT, Hsieh EW, et al. Accuracy of Blood Loss Measurement during Cesarean Delivery. AJP Rep 2017;7:e93-100. [Crossref] [PubMed]
- Blank DA, Polglase GR, Kluckow M, et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch Dis Child Fetal Neonatal Ed 2018;103:F539-46. [Crossref] [PubMed]
- Agarwal S, Jaiswal V, Singh D, et al. Randomised control trial showed that delayed cord clamping and milking resulted in no significant differences in iron stores and physical growth parameters at one year of age. Acta Paediatr 2016;105:e526-30. [Crossref] [PubMed]
- Jaiswal P, Upadhyay A, Gothwal S, et al. Comparison of Umbilical Cord Milking and Delayed Cord Clamping on Cerebral Blood Flow in Term Neonates. Indian J Pediatr 2015;82:890-5. [Crossref] [PubMed]
- Jaiswal P, Upadhyay A, Gothwal S, et al. Comparison of two types of intervention to enhance placental redistribution in term infants: randomized control trial. Eur J Pediatr 2015;174:1159-67. [Crossref] [PubMed]
- Yadav AK, Upadhyay A, Gothwal S, et al. Comparison of three types of intervention to enhance placental redistribution in term newborns: randomized control trial. J Perinatol 2015;35:720-4. [Crossref] [PubMed]
- Panburana P, Odthon T, Pongmee P, et al. The Effect of Umbilical Cord Milking Compared with Delayed Cord Clamping in Term Neonates: A Randomized Controlled Trial. Int J Womens Health 2020;12:301-6. [Crossref] [PubMed]
- Vatansever B, Demirel G, Ciler Eren E, et al. Is early cord clamping, delayed cord clamping or cord milking best? J Matern Fetal Neonatal Med 2018;31:877-80. [Crossref] [PubMed]
- Finn D, Ryan DH, Pavel A, et al. Clamping the Umbilical Cord in Premature Deliveries (CUPiD): Neuromonitoring in the Immediate Newborn Period in a Randomized, Controlled Trial of Preterm Infants Born at <32 Weeks of Gestation. J Pediatr 2019;208:121-6.e2. [Crossref] [PubMed]
- Katheria A, Reister F, Essers J, et al. Association of Umbilical Cord Milking vs. Delayed Umbilical Cord Clamping With Death or Severe Intraventricular Hemorrhage Among Preterm Infants. JAMA 2019;322:1877-86. [Crossref] [PubMed]
- Katheria AC, Truong G, Cousins L, et al. Umbilical Cord Milking Versus Delayed Cord Clamping in Preterm Infants. Pediatrics 2015;136:61-9. [Crossref] [PubMed]
- Rabe H, Jewison A, Alvarez RF, et al. Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates: a randomized controlled trial. Obstet Gynecol 2011;117:205-11. [Crossref] [PubMed]
- Shirk SK, Manolis SA, Lambers DS, et al. Delayed clamping vs. milking of umbilical cord in preterm infants: a randomized controlled trial. Am J Obstet Gynecol 2019;220:482.e1-e8. [Crossref] [PubMed]
- Barboza JJ, Albitres-Flores L, Rivera-Meza M, et al. Short-term efficacy of umbilical cord milking in preterm infants: systematic review and meta-analysis. Pediatr Res 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Katheria A, Garey D, Truong G, et al. A Randomized Clinical Trial of Umbilical Cord Milking vs. Delayed Cord Clamping in Preterm Infants: Neurodevelopmental Outcomes at 22-26 Months of Corrected Age. J Pediatr 2018;194:76-80. [Crossref] [PubMed]
- El-Naggar W, Afifi J, Dorling J, et al. A Comparison of Strategies for Managing the Umbilical Cord at Birth in Preterm Infants. J Pediatr 2020;225:58-64.e4. [Crossref] [PubMed]
- Katheria AC, Reister F, Hummler H, et al. LB 1: Premature Infants Receiving Cord Milking or Delayed Cord Clamping: A Randomized Controlled Non-inferiority Trial. Am J Obstet Gynecol 2019;220:S682. [Crossref]
- Atiam H. The Hematologic Impact of Umbilical Cord Milking Versus Deferred Cord Clamping in Premature Neonates. 2020. Available online: ClinicalTrials.gov Identifier: NCT03147846. Accessed June 26, 2020.
- Al-Wassia H. Deferred Cord Clamping Compared to Umbilical Cord Milking in Preterm Infants. Accessed June 27, 2020.
- Cetinkaya M. The Effect of Delayed Cord Clamping and Milking on the Amount of Stem Cells In Preterm. Available online: ClinicalTrials.gov Identifier: NCT04057027. Accessed June 27, 2020.
Cite this article as: Govindaswami B, Nudelman M, Jegatheesan P, Song D. A narrative review of delaying cord clamping 2020—who, what, when, where, why and how? Pediatr Med 2020;3:25.

