Antenatal diagnosis of biliary atresia: a narrative review
Introduction
Biliary atresia (BA) is an obliterative disease of the extra- and intrahepatic biliary tree that represents the leading indication for liver transplantation in childhood (1,2). This disease usually presents as persistent jaundice beyond the first few weeks of life (3). Nevertheless, 10–15% of patients present a developmental and truly innate disease in origin, showing either congenital anomalies (such as those in biliary atresia splenic malformation syndrome, BASMs) or cystic dilatation of the extrahepatic biliary tract that takes origin in early embryogenic life (2,4). The importance of these variants is two-fold: first, understanding their aetiopathogenesis, so closely connected with the biliary ducts development, could possibly explain part of this largely unknown disease. Second, antenatal suspicion of BA could target the post-natal diagnostic assessment of a cholestatic neonate, avoiding delay in diagnosis and surgical intervention, as the early diagnosis of BA is associated with a better outcome after portoenterostomy (1). Since the first report of antenatal diagnosis of BA by Tsuchida et al. (5), only few case reports and small series have been described, and the impact of antenatal diagnosis on clinical outcome is largely unknown (3,6-8). The present work provides a detailed review of antenatal diagnosis of BA, focusing on the ultrasonographic findings of non-visualization of the gallbladder (GB), cystic dilatation of the extrahepatic biliary tract and congenital anomalies associated with BA. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/pm-20-60).
Methods
This review was performed according to preferred reporting items for reviews and guidelines (9). We searched for original studies on prenatal diagnosis of BA published since 1985 from PubMed and Medline database. Eligible study designs were case report, case series and review. We omitted articles where antenatal diagnosis was not clearly specified and papers not written in English. We then evaluated the full text of the selected articles. The date of the last search was June 2020.
Non-visualization of the GB
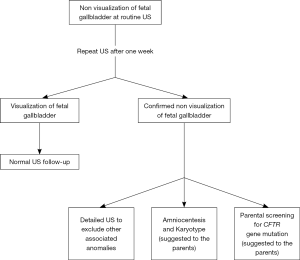
In the last two decades, the remarkable advances in ultrasonography have led to the possibility of a better antenatal evaluation of the biliary tree and, in particular, of the GB. The GB is derived from the ventral outgrowth of the foregut caudal portion around the fourth week of gestation (10). The initial diverticulum gradually develops into the GB and the cystic duct and it is located at a 30- to 45-degree angle to the right of the umbilical vein and its size increases during pregnancy, reaching a plateau at around 30 weeks (10,11). GB can be detected during the antenatal scan performed from the 14th-16th week of gestation. Visualization of the fetal GB has been reported in 55–82% of transabdominal US in the second trimester and in 99% of transvaginal US (7,10,11). Non-visualization of fetal gallbladder (NVFGB) is a rare condition, reported in 1 out of 875 pregnancies (0.1%) (7,12,13). NVFGB is defined as non-visualization of the organ on two targeted ultrasound examinations performed within a week period (14). As demonstrated by Dreux et al., the GB was then demonstrated in 16% of NVFGB cases prenatally at routine third-trimester scan and at birth in 37% of cases (15). The outcome of fetuses with NVFGB, confirmed at birth, can differ greatly between a benign condition, such as isolated GB agenesis, and severe diseases like BA and cystic fibrosis (CF) (7). In case of NVFGB it is mandatory to exclude the presence of additional ultrasound abnormalities such as polysplenia, hyperechogenic bowel and cardiac anomalies, which would significantly increase the risk of chromosomal abnormalities, BA and cystic fibrosis (14,15). Dreux et al. reported a large cohort of fetuses with NVFGB, at a median gestational age of 23 weeks; in this study, 17 out of 102 fetuses (16.7%) presented additional abnormalities (chromosomal or structural defects) and in the totality of these cases a severe condition was then discovered at birth (15). In this subgroup, BA was subsequent diagnosed in 17% of the neonates. Conversely, in 85 fetuses NVFGB was an isolated finding; at birth, only 9.4% of fetuses of this subgroup presented a severe disease and BA was subsequently diagnosed in 5.8%. The remaining 90.6% of patients were healthy infants at birth. These data were further confirmed in the meta-analysis provided by Di Pasquo and colleagues; in this study, among the 170 euploid fetuses with isolated NVFGB, BA was confirmed after birth in 4.8% of cases (14). Shen et al. calculated a probability of BA at around 3% when NVFGB is confirmed on repeated ultrasound evaluations (12). Moreover, they have also retrospectively reviewed prenatal ultrasound scans of 25 patients with post-natal diagnosis of BA, reporting in 6 of them (24%) an absent or an abnormal GB (12). All these studies concluded that NVFGB confirmed in the second trimester US is associated with severe anomalies in up to a quarter of patients, even if the risk of BA is much lower. Genetic consultation and amniocentesis for karyotyping should be therefore offered to the parents and this assessment is routinely performed in the majority of Western Centres (7,15,16). When the NVFGB is confirmed, and in particular when the suspicion of CF is high (e.g., positive familiar history, hyperechogenic bowel or meconium peritonitis), CF gene mutation screening should be proposed to the parents. Second level fetal RMN assessment, unlike other congenital anomalies, is not usually necessary. A practical diagnostic algorithm is resumed in Figure 1 (7,10,11,17).
Cystic dilatation of the extrahepatic biliary tract
Cystic biliary atresia (CBA) is an uncommon form of BA with an occurrence rate of 5–10% of all BA cases (3,18,19), even if incidence in some Japanese series is up to 20% (19). Patients with this variant present BA and cystic dilatation of any part of the extrahepatic biliary tract, containing either clear fluid or bile, depending on whether communication with the intrahepatic biliary system persists (18,19). This BA variant occurs relatively late in gestation beyond the period of initial bile production at 12 weeks with at least some luminal integrity between intrahepatic and extrahepatic systems (19). As reported by Caponcelli et al., CBA is a clinically distinct variant of BA that presents definite features including early age at diagnosis and predictable response to surgery, mainly related to age (18). Moreover, this better outcome after surgery is related to the more mature intrahepatic bile system. The King’s College series presented a strong evidence of the antenatal origin of CBA, along with BASM syndrome, with a prenatal US detection rate up to 40% (18). In prenatal diagnosis, CBA may lead to diagnostic confusion with congenital choledochal malformation (CM), as both CBA and CM can be suspected when a cyst at the porta hepatis is observed (3). Both these different diseases, clinically defined as “biliary tree cystic malformations” (BCM), are extremely uncommon, even if progress in prenatal ultrasonography and improved new technologies can allow their identification especially during the second and third trimester of pregnancy, between the 15th and 34th gestational week (19). Many studies have questioned the possibility of prenatally distinguish CBA from CM (3,8,20). Efforts have been made to highlight the potential different US patterns of these different diseases, in the attempt to be more specific in prenatal diagnosis (11,21,22). Casaccia et al. reported a series of 5 prenatally diagnosed subhepatic cysts, emphasizing that variations of US pattern and change in size during pregnancy allow to make differential diagnosis (21). In this study, anechoic, static and small cysts in the hepatic hilum were highly suspicious for BA, whilst echoic ones were suggestive of CM. Moreover, large or enlarging echoic cyst could represent an early obstructed CM. The constancy of the cyst size and volume in BA is thought to be due to insufficient bile excretion into the common bile duct (CBD) (5,23,24). Conversely, in cases of CM, unless the distal CBD is occluded, the cyst size may change and the mass grow as pregnancy progresses (24). Certainly, the evaluation of the GB is mandatory (3). The US appearance of GB and its connection with the cyst has been suggested as highly useful: the absence of GB or the presence of an irregular, dysmorphic, elongated GB could indicate CBA. All these features may also help ruling out other non-biliary diseases associated with a prenatal US finding of cystic mass in the abdomen, including duodenal atresia or duodenal duplication, ovarian cyst, liver cyst, mesenteric cyst and hydronephrosis (23). Differentiating CBA from CM remains difficult and to date there is still no unequivocally accepted US sign allowing the clinicians to recognize CBA from CM, as they frequently share the same appearances. Due to this lack of uniformity, Davenport had also suggested an algorithm of management of antenatally diagnosed BCM (3). If dilatation of the intrahepatic biliary ducts is assessed, the diagnosis of CBA may potentially be ruled out. Furthermore, using colour Doppler US, dilated intrahepatic vascular system can be discerned from dilatation of the bile duct.
In order to elucidate the exact origin of BCM, other types of imaging such as 3D-US, MRI and fetal MR cholangiography have been proposed (25-27). Recent development of ultrafast MR imaging techniques has allowed MRI to become an important complement to US in fetal imaging. It is relatively operator independent and can offer excellent anatomical and contrast resolutions, and larger field of view (28). Although 3D-US and MRI may be helpful in prenatal diagnosis and give additional information, standard 2D-US is sufficient to evaluate and examine the fetal biliary system in most cases. Moreover, due to its real-time capability, non-invasiveness, availability, and low-cost, US remains the primary and most important imaging modality for prenatally detecting CBA.
Strict differentiation between CBA and CC is still very arduous and challenging and remains the key clinical problem. Davenport et colleagues concluded that no preoperative imaging studies can conclusively discriminate CBA from CMs, hence surgical inspection and intra-operative cholangiography are crucial (3,18).
Associated congenital anomalies
The extrahepatic bile duct begins as a diverticulum from the duodenum around 20 days’ gestation, before that any intrahepatic duct is present. The formation of the extrahepatic ducts is lined by cholangiocytes expressing transcription factors common to the pancreas and duodenum (e.g., PDX-1, PROX-1, HNF-6) (19,29). This occurs in parallel with key changes in visceral rotation, cardiac and splenic development, and evolution of the portal and inferior vena cava. Bile duct mal-development at this stage could explain the Biliary Atresia Splenic Malformation (BASM) syndrome, a subset of BA that encompasses splenic malformations, visceral symmetry defects, cardiac and intra-abdominal veins malformations (4,30).
BASM has been reported in Western series with an incidence rate of 10–15%, while in Eastern Countries this subset of BA involves 5% of BA patients, suggesting a wide geographical distribution of this particular subgroup (31,32).
These infants are usually girls, and some seem to come from an abnormal intrauterine environment (e.g., maternal diabetes and thyrotoxicosis). BASM associated malformations include (4):
- Splenic anomalies: polysplenia, double spleen and asplenia;
- Laterality defects: situs viscerum inversus, dextrocardia, heterotaxia, central-positioned liver and gut malrotation;
- Vascular anomalies: preduodenal portal vein, absence of portal vein with a mesenteric-hemiazygos shunt and arterialized liver and absence of inferior vena cava;
- Structural cardiac diseases: ventricular septal (VSD) and/or atrial septal defects (ASD), tetralogy of Fallot, aortic arch abnormalities, hypoplastic left heart, left atrial isomerism, pentalogy of Cantrell.
Some of these patients had overlap anomalies such as sacral agenesis and double-outlet right ventricle, markers of a diabetic embryopathy (4). Some features of BASMs, such as polysplenia, laterality defects or cardiac malformations, could be easily found during routine US screening during pregnancy. Nevertheless, considering the low incidence of BASM, only few studies reported antenatal detection of this BA form (33). Few studies concluded that prenatally diagnosed left isomerism is associated with BA in up to 10% of cases: it would appear that an association between this finding and a small or absent GB could be highly suggestive of BASM (12,33,34).
BASM syndrome appears to be a distinct subgroup in infants with BA. Postnatally, these patients typically have no CBD, a tiny atrophic GB, and the liver parenchyma appears entirely normal at birth (4,35,36). These patients tend to have a worse prognosis than isolated BA; nevertheless, it is unclear whether this is mainly caused by the burden of associated congenital anomalies. Careful follow-up should be guaranteed for these patients because of a potentially higher risk of post-operative complications such as hepatopulmonary syndrome (HPS) (4,32).
Eventually, there are other syndromic associations which are not listed in the BASM spectrum, including the so-called cat-eye syndrome (coloboma, anorectal atresia, and chromosome 22 aneuploidy) (37). Moreover, some infants with BA have associated anomalies such as esophageal atresia, jejunal atresia, anorectal malformations, horseshoe kidney, ciliary dyskinesia, caudal regression syndrome (38-41). Main associated anomalies that could be detected prenatally are resumed in Table 1.
Table 1
| BASM syndrome |
| Splenic anomalies: polysplenia, double spleen, asplenia |
| Laterality defects: situs viscerum inversus, dextrocardia, heterotaxia, central-positioned liver and gut malrotation |
| Vascular anomalies: preduodenal portal vein, absence of portal vein with a mesenteric-hemiazygos shunt and arterialized liver and absence of inferior vena cava |
| Structural cardiac diseases: ventricular septal (VSD) and/or atrial septal defects (ASD), tetralogy of Fallot, aortic arch abnormalities, hypoplastic left heart, left atrial isomerism, pentalogy of Cantrell |
| Cat-eye syndrome |
| Chromosome 22 aneuploidy, trisomy, duplication |
| Total pulmonary venous return anomaly, tetralogy of Fallot |
| Absence of one or both kidneys, hydronephrosis, supernumerary kidneys, and/or renal hypoplasia |
| Spinal defects and limb malformations |
| Intestinal malrotation, rectal atresia |
| Isolated anomalies |
| Esophageal atresia, intestinal atresia, caudal regression syndrome, cystic kidney, hydronephrosis, vertebral and rib anomalies, congenital diaphragmatic hernia |
Conclusions
The aetiology and pathogenesis of BA are still largely unknown. Nevertheless, a small subset of patients presents a clearly developmental disease that origins in embryogenic life. Some different features of BA could be detected on antenatal routine US in different gestational age, including non-visualization of the fetal GB, cystic dilatation of the extrahepatic biliary tract and associated congenital anomalies such as those in BASMs. The impact of antenatal diagnosis on surgical outcome is still largely unknown. On one hand, an early and accurate post-natal assessment avoids diagnostic and surgical delays, may lead to early diagnosis and early portoenterostomy with associated better outcome. This is particularly true for cystic and BASM subgroup. On the other hand, the effect of age at portoenterostomy for patient with isolated BA (the majority of cases) is still controversial. Moreover, early post-natal liver biopsies may not display characteristic findings of BA. Antenatal suspicion of BA can target the post-natal assessment of cholestatic neonates, promoting centralization of the family and a more accurate diagnosis.
Future research should focus on understanding the etiopathogenesis of these prenatal forms of BA, so closely connected with biliary ducts development, in order to explain part of this still largely unknown disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/pm-20-60
Conflicts of Interest: All the authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-60). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parolini F, Boroni G, Milianti S, et al. Biliary atresia: 20-40-year follow-up with native liver in an Italian centre. J Pediatr Surg 2019;54:1440-4. [Crossref] [PubMed]
- Frassetto R, Parolini F, Marceddu S, et al. Intrahepatic bile duct primary cilia in biliary atresia. Hepatol Res 2018;48:664-74. [Crossref] [PubMed]
- Davenport M, Hadzic N. Prenatal diagnosis of liver and biliary tract disease. Semin Neonatol 2003;8:347-55. [Crossref] [PubMed]
- Davenport M, Tizzard SA, Underhill J, et al. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006;149:393-400. [Crossref] [PubMed]
- Tsuchida Y, Kawarasaki H, Iwanaka T, et al. Antenatal diagnosis of biliary atresia (Type I cyst) at 19 weeks gestation: differential diagnosis and etiologic implications. J Pediatr Surg 1995;30:697-9. [Crossref] [PubMed]
- Hinds R, Davenport M, Mieli-Vergani G, et al. Antenatal presentation of biliary atresia. J Pediatr 2004;144:43-6. [Crossref] [PubMed]
- Shen O, Rabinowitz R, Yagel S, et al. Absent gallbladder on fetal ultrasound: prenatal findings and postnatal outcome. Ultrasound Obstet Gynecol 2011;37:673-7. [Crossref] [PubMed]
- Matsubara H, Oya N, Suzuki Y, et al. Is it possible to differentiate between choledochal cyst and congenital biliary atresia (type I cyst) by antenatal ultrasonography? Fetal Diagn Ther 1997;12:306-8. [Crossref] [PubMed]
- Green BN, Johnson CD, Adams A. Writing Narrative Literature Reviews for Peer-Reviewed Journals: Secrets of the Trade. J Chiropr Med 2006;5:101-17. [Crossref] [PubMed]
- Chan L, Rao BK, Jiang Y, et al. Fetal Gallbladder Growth and Development During Gestation. J Ultrasound Med 1995;14:421-5. [Crossref] [PubMed]
- Goldstein I, Tamir A, Weisman A, et al. Growth of the Fetal Gall Bladder in Normal Pregnancies. Ultrasound Obstet Gynecol 1994;4:289-93. [Crossref] [PubMed]
- Shen O, Sela HY, Nagar H, et al. Prenatal diagnosis of biliary atresia: a case series. Early Human Development 2017;111:16-9. [Crossref] [PubMed]
- Blazer S, Zimmer EZ, Bronshtein M. Nonvisualisation of the fetal gallbladder in early pregnancy: comparison with clinical outcome. Radiology 2002;224:379-82. [Crossref] [PubMed]
- Di Pasquo E, Kuleva M, Rousseau A, et al. Outcome of non-visualization of fetal gallbladder on second-trimester ultrasound: cohort study and systematic review of literature. Ultrasound Obstet Gynecol 2019;54:582-8. [Crossref] [PubMed]
- Dreux S, Boughanim M, Lepinard C, et al. Relationship of non-visualization of fetal gallbladder and amniotic fluid digestive enzymes analysis to outcome. Prenatal Diagnosis 2012;32:423-6. [Crossref] [PubMed]
- Ben-Ami M, Perlitz Y, Shalev S, et al. Prenatal diagnosis of extrahepatic biliary duct atresia. Prenat Diagn 2002;22:583-5. [Crossref] [PubMed]
- Koukoura O, Kelesidou V, Delianidou M, et al. Prenatal sonographic diagnosis of biliary tract malformations. J Clin Ultrasound 2019;47:292-7. [Crossref] [PubMed]
- Caponcelli E, Knisely AS, Davenport M. Cystic biliary atresia: an etiologic and prognostic subgroup. J Pediatr Surg 2008;43:1619-24. [Crossref] [PubMed]
- Parolini F, Davenport M. Biliary atresia - new developments. In: Lima M, Reimberg O. editors. Neonatal Surgery - Contemporary Strategies from Fetal Life to the First Year of Age. Cham, Switzerland: Springer International Publishing, 2019:381-400.
- Komuro H, Makino SI, Momoya T, et al. Biliary atresia with extrahepatic biliary cysts - Cholangiographic patterns influencing the prognosis. J Pediatr Surg 2000;35:1771-4. [Crossref] [PubMed]
- Casaccia G, Bilancioni E, Nahome A, et al. Cystic anomalies of biliary tree in the fetus: Is it possible to make a more specific prenatal diagnosis? J Pediatr Surg 2002;37:1191-4. [Crossref] [PubMed]
- Morel B, Kolanska K, Dhombres F, et al. Prenatal ultrasound diagnosis of cystic biliary atresia. Clin Case Rep 2015;3:1050-1. [Crossref] [PubMed]
- Schroeder D, Smith L, Prain HC. Antenatal diagnosis of choledochal cyst at 15 weeks’ gestation: Etiologic implications and management. J Pediatr Surg 1989;24:936-8. [Crossref] [PubMed]
- Hasegawa T, Sasaki T, Kimura T, et al. Prenatal ultrasonographic appearance of type IIId (uncorrectable type with cystic dilatation) biliary atresia. Pediatr Surg Int 2002;18:425-8. [Crossref] [PubMed]
- Lee IH, Kim GJ. Fetal choledochal cyst diagnosed at 22 weeks of gestation by three-dimensional ultrasonography: A case report. J Korean Med Sci 2008;23:909-11. [Crossref] [PubMed]
- Quinn TM, Hubbard AM, Adzick NS. Prenatal magnetic resonance imaging enhances fetal diagnosis. J Pediatr. Surg 1998;33:553-8. [Crossref] [PubMed]
- Nori M, Venkateshwarlu J, Vijaysekhar V, et al. Extrahepatic biliary atresia with choledochal cyst: Prenatal MRI predicted and post natally confirmed: A case report. Indian J Radiol Imaging 2013;23:238-42. [Crossref] [PubMed]
- Wong AMC, Cheung YC, Liu YH, et al. Prenatal diagnosis of choledochal cyst using magnetic resonance imaging: A case report. World J Gastroenterol 2005;11:5082-3. [Crossref] [PubMed]
- Davenport M. Biliary atresia: From Australia to the zebrafish. J Pediatr Surg 2016;51:200-5. [Crossref] [PubMed]
- Guttman OR, Roberts EA, Schreiber RA, et al. Biliary atresia with associated structural malformations in Canadian infants. Liver Int 2011;31:1485-93. [Crossref] [PubMed]
- Zhan J, Feng J, Chen Y, et al. Incidence of biliary atresia associated congenital malformations: A retrospective multicenter study in China. Asian J Surg 2017;40:429-33. [Crossref] [PubMed]
- Nio M, Wada M, Sasaki H, et al. Long-term outcomes of biliary atresia with splenic malformation. J Pediatr Surg 2015;50:2124-7. [Crossref] [PubMed]
- Gottschalk I, Stressig R, Ritgen J, et al. Extracardiac anomalies in prenatally diagnosed heterotaxy syndrome. Ultrasound Obstet Gynecol 2016;47:443-9. [Crossref] [PubMed]
- Escobar-Diaz MC, Friedman K, Salem Y, et al. Perinatal and infant outcomes of prenatal diagnosis of heterotaxy syndrome (asplenia and polysplenia) Am J Cardiol 2014;114:612-7. [Crossref] [PubMed]
- Makin E, Quaglia A, Kvist N, et al. Congenital biliary atresia: liver injury begins at birth. J Pediatr Surg 2009;44:630-3. [Crossref] [PubMed]
- Davenport M, Savage M, Mowat AP, et al. Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery 1993;113:662-8. [PubMed]
- Allotey J, Lacaille F, Lees MM, et al. Congenital bile duct anomalies (biliary atresia) and chromosome 22 aneuploidy. J Pediatr Surg 2008;43:1736-40. [Crossref] [PubMed]
- Gupta L, Bhatnagar V. A study of associated congenital anomalies with biliary atresia. J Indian Assoc Pediatr Surg 2016;21:10-3. [Crossref] [PubMed]
- Schwarz KB, Haber BH, Rosenthal P, et al. Extra-hepatic anomalies in infants with biliary atresia: results of a large prospective North American multi-center study. Hepatology 2013;58:1724-31. [Crossref] [PubMed]
- Tyraskis A, Davenport M. Steroids after the Kasai procedure for biliary atresia: the effect of age at Kasai portoenterostomy. Pediatr Surg Int 2016;32:193-200. [Crossref] [PubMed]
- Lemoine C, Melin-Aldana H, Brandt K, et al. The evolution of early liver biopsy findings in babies with jaundice may delay the diagnosis and treatment of biliary atresia. J Pediatr Surg 2020;55:866-72. [Crossref] [PubMed]
Cite this article as: Parolini F, Pecorelli S, Stern MV, Montanaro B, Siano E, Boroni G, Alberti D. Antenatal diagnosis of biliary atresia: a narrative review. Pediatr Med 2020;3:10.