Open-surgery repair of congenital malformation of the chest: indications, technical tips and outcomes
Introduction
Congenital malformations of the chest encompass a wide spectrum of deformities which require different approaches depending on the specific type and degree of functional impairment.
The interest for the correction of these anomalies has increased dramatically in the last 20 years, and the treatment has undergone enormous development in the same period.
In pediatric patients, different scenarios can be observed:
- Severe congenital deformities of the chest wall, with respiratory impairment, requiring early surgical treatment. This is a very uncommon scenario, but it can happen in severe Poland syndrome (PS) or in asphyxiating thoracic dysplasia (ADT) (Jeune syndrome);
- Congenital asymptomatic deformities, such as the vast majority of moderate to severe pectus excavatum (PE) and PS. These patients are evaluated by Pediatricians and may be candidates to non-invasive corrective treatments or simply followed up until puberty, while a surgical treatment (usually minimally invasive) is seldom required;
- Deformities not evident or not recognized or very mild in infancy that during pubertal growth become very evident and may require surgical treatment. This is a common scenario for pectus carinatum (PC) and mild to moderate PE. These are patients who can be asymptomatic or mildly symptomatic but they are usually very concerned by the deformity, especially for cosmetically related reasons.
With the introduction of thoracoscopic and minimally invasive repair of both PE and PC, and the implementation of non-invasive approaches to these anomalies (such as the vacuum bell and the FMF dynamic compression system), the open surgery, the only option in the last century, has nowadays become much less attractive in pediatric thoracic surgery, but it still represents the best approach to a few of the conditions that we will be dealing with in this chapter: Currarino-Silverman (CS) deformity, PS (and isolated costal agenesis), sternal cleft, Jeune syndrome. We are not describing open surgery for those conditions (PE, PC) for which a minimally invasive or noninvasive approach is preferable in pediatric age.
Methods
The authors describe the open surgery for congenital chest wall anomalies based on their dedication to this field of pediatric and thoracic surgery in national referral Centers where they have gained long-standing personal experience. The most common practice for both authors in the treatment of pediatric chest wall anomalies is represented by minimally invasive repair of PE with the Nuss technique that will not be dealt with here as the chapter focuses on open surgical treatment. For the same reason, all non-invasive treatments that are frequently adopted by the authors for their pediatric patients will not be described.
The following few indications to open surgical repair remain in pediatrics and will be the topic of our chapter:
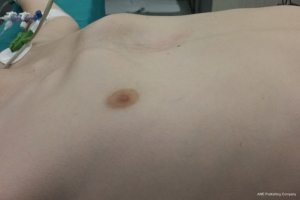
- CS deformity (1): also known as pectus arcuatum or pouter pigeon breast, is a combination of cartilage and sternal anomalies (Figure 1). The hallmark of this deformity is the bony fusion of the manubrium and sternal body, and anS-shaped lateral profile of the sternum, that produces a carinatum-like deformity superiorly and an excavatum inferiorly. Prominent cartilages (2nd to 4th) are usually associated.
- PS (2): the thoracic defect can be variably compromised. According to the Romanini et al. classification (Table 1), 4 degrees can be observed (3,4). T4 cases require tailored surgical repair with an open approach, though better with a multidisciplinary cooperation between pediatric thoracic and plastic surgeons.
Table 1
Romanini et al. classification for thoracic defect in PST: thoracic anomaly T1: pectoralis muscle aplasia (partial or complete) T2: T1 and pectus carinatum/excavatum T3: T1 and costal agenesis T4: T1 + T2 + T3 B: breast anomaly B1: breast hypoplasia B2: breast aplasia N: nipple areola complex (NAC) N1: NAC hypoplasia with dislocation less than 2 cm N2: NAC hypoplasia with dislocation more than 2 cm N3: absent NAC PS, Poland syndrome. - Sternal clefts (5): this congenital anomaly is the only one that would require a neonatal repair. In the case of missed repair, later surgery will present more difficulties and can require different approaches.
- Jeune syndrome (ADT) (6): though extremely uncommon, this is the most severe congenital thoracic anomaly, leading to fatal respiratory insufficiency if not treated in severe variant.
Results
CS deformity (1), also called pouter pigeon breast or pectus arcuatum (Figure 1)
There is still some confusion in medical reports about this condition, which is not well-known and should be differentiated from PC and from PE because of the following unique features:
- While in PE and PC the “primum movens” of the anomaly resides in the cartilages, in CS deformity the sternum itself is shorter and deformed: due to premature fusion of the manubrium with the corpora, the sternal profile is typically S-shaped, with a carinatum-like deformity in the upper moiety and an excavatum-like aspect in the lower one.
- Anomalous prominence usually involving three cartilage ribs on each side (usually from 2nd to 4th rib) is associated to the sternal anomaly, giving these patients’ anterior chest wall the aspect of an arch (pectus arcuatum).
- The depression of the inferior half of the sternum may be more or less evident [according to some authors (7) differentiating type A or B of CS deformity], but it is usually not as severe as to give cardiac compression.
- In CS deformity, cardiac intrinsic anomalies are more frequent than in the general population and in other chest wall deformities, so cardiac ultrasound is mandatory before the correction.
- As far as we know, CS deformity is not a congenital condition, usually appearing during school age and becoming more severe after around 12 years of age.
- Symptoms can be present (respiratory mild disturbances especially during strenous activity or some degree of pain) but in most patients the main concern is represented by the poor cosmetical appearance of these thoraces.
- For reasons expressed in point (I), non-invasive treatments are not indicated for CS deformity: neither vacuum bell nor dynamic compression system can be effective treatments for this condition. Also minimally invasive approaches commonly used for PE and PC correction are not effective for CS deformity repair.
The indications to surgical correction are based on symptoms if present, but in any case on cosmetical reasons. In our experience, the best age for the correction is just at the beginning of puberty, when the deformity is still quite malleable, although this deformity may also be successfully repaired in adults. It is not usually necessary to operate on patients at earlier ages, when surgery can be performed easily, but it is more at risk to give rib cage growth impairment or recurrences.
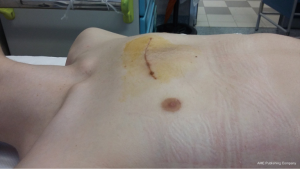
The classical open approach for CS deformity repair (Figure 2) is a sternochondroplasty according to Ravitch principles (8). A number of variations of the Ravitch techniques for PC and PE can be applied to CS deformity repair (9-13). The main principles are:
- Sub-perichondral resection of the prominent anomalous cartilages;
- Wedge osteotomy of the sternum, that can be single at the level of the junction between the manubrium and the body, or double, by adding another incision to it at the level of the upper level of sternal depression;
- Treating the depression by either raising the sternum itself or by filling the defect with autologus material.
Sub-perichondral multiple resections: through a horizontal or vertical incision, carried out at the level of the sternal defect, pectoralis major muscles are exposed. Depending on the number of cartilage rib affected to be removed, the pectoralis major muscles are detached from the sternum or just incised and separated in order to expose the ribs. In adolescents, a segment of cartilage rib to be removed is usually calculated leaving the growth ossification center intact at the level of bone to cartilage junction. This precaution is unnecessary for adults. The anterior perichondrium is opened and the cartilage is surrounded by dissecting intra-perichondrally with blunt instruments. Different instruments or tips can be adopted, such as a mosquito gently pushed around the cartilage rib sometimes with the help of a gauze to detach the cartilage from the posterior perichondrium more easily are commonly used. A dovetail dissector has been designed by Welch for this maneouvre and we found it very useful to perform a quick and safe dissection. The main pitfalls of this maneouvre are to enter the pleura by dissecting too deeply, enter the cartilage by dissecting too superficially, or injure the internal thoracic or intercostal vessels. The surgeon should decide precisely how many cartilages to remove as well as how much length.
Osteotomies: they can be complete through both sternal plates and just the anterior plate. The shape of the osteotomy should be decided by removing the bony anterior angle completely, which is so important in determining the deformity. An oscillating saw is usually the preferred instrument to achieve a quick osteotomy. In minor cases or with younger patients a bone removal forceps can achieve the same result. Digital retrosternal dissection can be useful to raise the sternum and perform a safe osteotomy by maintaining a tactile sensation posteriorly. Wax or cartilage-bone chips may be useful to reduce bleeding from osteotomies. After osteotomy, the two edges of the sternum can be approximated with steel wires or titanium plates, thus obtaining a straight sternal body.
To correct the depression of the lower half of the sternum there are different options. A possible cardiac compression has to be evaluated before surgery through CT scan or MRI. In case of cardiac compression, this has to be relieved. The sternum can be lifted up with the help of a second inferior osteotomy and pushing it upwards after dividing the xiphoid process and dissecting retrosternally. Alternatively, either a prolene mesh may be passed retro-sternally, inserted on the lateral rib stumps and pulled tight (in adults) or a retrosternal bar can be used; the latter is inserted thoracoscopically according to the Nuss technique. We have tried all techniques: the Nuss bar is a good alternative to keep the surgical access smaller, but it is important to underline that the Nuss bar alone (without cartilage resections and osteotomy) is not able to correct a CS deformity with a good result.
In case of mild sternal depression without cardiac compression, we have developed a less invasive technique over the last few years, which consists in filling the depression with autologous cartilage and bone pieces derived from cartilage resection and osteotomies. The cartilage is very easy to divide into little particles that can fill the defect precisely. To maintain the cartilage pieces in place, we insert them into a subcutaneous xhyphoid pocket, wrapped inside a Vicryl mesh.
It is important to drain the subcutaneous tissue at the end of the procedure in order to avoid post-operative collections.
In our experience we have observed that autologous material can undergo very little absorption during the following months.
PS (2)
PS is a rare and poorly known congenital anomaly, which is sporadic in the majority of cases and in all cases involves the pectoralis major muscle, breast, and subcutaneous tissue of the pectoralis area; in some cases associated with upper arm and/or rib defect. There is a wide range of severity of PS, as described by Romanini et al., who classified the thoracic defect in 4 degrees of severity, from T1 to T4 (3,4). In this chapter we will treat only cases with severe thoracic defect, requiring surgical repair, corresponding to T2, T3 or T4 of the Romanini classification.
To classify the defect the clinical evaluation is sufficient, but CT scan with 3D reconstruction is required to plan the surgical approach.
Surgical treatment of PS is performed mainly for two indications: anterior chest wall anomaly (similar to PE, PC or more frequently a combination of both) and rib agenesis with antero-lateral defect, paradoxical respiratory movements and lung herniation.
It is not clear why in some PS patients, anterior chest wall deformity occurs, sometimes severe, and in others it does not. In most cases, more than a true PE, or PC, there is a combination of both, due to some degree of sternal rotation towards the defect side. In these patients, the chest wall is depressed on the same side of PS and is prominent on the opposite side. In other patients, a CS-like deformity is observed. In all cases of PS with anterior chest wall anomaly, the indication is mainly cosmetical. In some PS patients with severe PE, compression of the lungs and or the heart can be a functional indication.
Regarding rib defect, depending on the number of ribs affected (usually lacking the anterior arch), some degree of lung herniation can occur. Respiratory dynamics can be anomalous but usually it does not cause any significant dysfunction, if the defect is limited to 1 to 3 ribs. Therefore, the indication to surgical repair is based on the concept of giving protection to the lungs and thoracic viscera. In our experience, it is not necessary to perform these operations until the beginning of pubertal growth.
In our experience, the two above-mentioned indications to rib cage repair can be addressed during the same surgical approach, sometimes associated with a plastic surgery step.
Usually, anterior chest wall anomalies are corrected through a midline short incision, giving access to the sternum and parasternal cartilages. A tailored approach is required. In most cases, the parasternal protruding cartilages on the opposite side of the PS defect should be removed, leaving the posterior perichondrium intact. Sometimes the same manoeuvre is required on the same side of PS defect. The sternum, if deformed, can be corrected through osteotomies.
The rib defect requires a tailored, reconstructive approach, through a second incision, carried out on the axillary line. The technique will vary, mainly according to the numbers of ribs affected (4): if only one rib is absent, no surgical repair is usually necessary. In the case of two ribs, the defect in our experience is well-fixed with a Goretex prosthesis, which gives enough stability to the rib cage. If the defect involves more than two ribs, we have been using metallic ribs as a bridge between the lateral rib stumps and the sternum. Titanium bars, that can be bent and cut during surgery, accordingly to the patient morphology of the chest defect, have been our preferred approach for a long time. These bars are screwed to the ribs and to the sternum with titanium screws. It is important to free a sufficient tract of rib laterally, in order to create the space for the screws and to get a segment of rib of enough thickness. The bars are then covered with a Goretex sheet, to avoid direct contact between the titanium and the skin, considering that in this area subcutaneous tissue is usually poorly represented.
With new materials and technologies available, older techniques such as costal transposition have been abandoned in our Institutions, as they were less effective (cartilage transposed could be reabsorbed later), required more skin incisions and could create a defect at the level of the harvesting site.
In recent years, we have been using custom-made metallic prosthesis, built on rib cage models obtained with 3D printing, based on CT scan or MRI of the chest. The same two incisions (pre-sternal on midline, and axillary) were necessary. The advantage of a customized prosthesis is that it fits the rib cage defect exactly, so all the adjustments that were necessary when using titanium bars are avoided. The prosthesis can be customized according to the surgeon’s preferences. We have found it useful to use a sternal plate that is screwed to the sternum; two metallic bars are inserted exactly into it, and they are again fixed to the sternum and to the anomalous ribs with other screws. The prosthesis is then covered by a Goretex sheet. The size, orientation of the prosthesis, the caliber and length of the screws are chosen based on the pre-operative imaging, which was impossible when using traditional metallic bars. In our experience this new approach makes the reconstructive surgery easier, and at the same time achieves better results. The only disadvantage of using customized prostheses is their higher cost. We usually perform a combined surgical approach together with plastic surgeons, so during the same surgery we repair the rib cage defect, the anterior chest wall deformity, and the soft tissue defect (muscular, nipple and breast defect). The plastic surgery is not the topic of this chapter, but in PS it is the key to obtaining a satisfactory cosmetic result. Briefly, our plastic surgeons have abandoned the use of the latissimus dorsi flap, as they found it more useful and less invasive to adopt a step by step procedure, using lipofilling, tissue expanders if necessary and finally pectoral or breast prostheses (4). In fact, latissimus dorsi is an important muscle of the back, therefore harvesting, rotating and placing it anteriorly, represents major surgery potentially leaving a significant impact on the back and shoulders in young patients. Conversely, lipofilling is much a less invasive technique that can be repeated and allows to fill cosmetical defects easily. Nowadays, in our experience we prefer to associate lipofilling to tissue expanders and breast or pectoral prostheses.
Regarding the timing of the corrective surgery, it has been demonstrated that a multi-step, minimally invasive corrective pathway starting at the beginning of puberty is advantageous in terms of good results and less psychological disturbances, with respect to a single large surgery at the end of puberty or in adult age (14).
Costal agenesis may occur also without the association with PS. These conditions are very uncommon and may require surgical repair basically for the same indications as PS: protection, respiratory paradoxical movements, cosmesis. The same principles and operations described for PS can be adopted in these patients, in whom the soft tissues are not involved, thus avoiding the need of an associated procedure of plastic surgery
Sternal cleft (5)
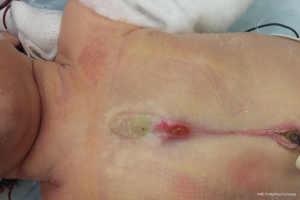
It is a rare anomaly, in which the mediastinal viscera are exposed without any protection (Figure 3). They can be total or partial, in this case more frequently involving the upper part of the sternum. Possibly associated with Cantrell pentalogy, PHACES syndrome or other anomalies (hemangiomas, congenital scars, skin lesions), they require repair in all cases, for the following reasons: protection, avoiding paradoxical respiration, improving cosmesis. A successful and relatively easy repair is better obtained during neonatal age. At this time, in fact, the two sternal halves can be usually approximated in the midline without difficulty, due to the compliance of thoracic bone and connective tissues. Before repair, cardiac and other anomalies have to be ruled out carefully.
To repair a sternal cleft in neonatal age, we usually perform a midline incision and we refresh the sternal edges with cautery. If the sternal cleft is partial, a V-shaped incision is performed where the normally fused sternum begins, in order to facilitate the primary closure. The sternal halves are tightened together with some unabsorbable stitches. When performing a primary repair of the sternum, it is important to be sure that the closure is not causing any hemodynamic troubles by diminishing the venous return, due to the dramatic increase in the mediastinal pressure. To reduce this risk, we advise to remove the thymus completely or partially (15). If the compression is too much or if the sternal halves cannot be approximated due to their poor compliance (as in older patients), multiple chondrotomies can be performed. The cartilages obtained may be used to reinforce the midline suture plan. A periosteal flap, obtained from each of the sternal bars, can be rotated and sutured on the midline, representing the posterior wall of the “new sternum”. Any remaining gap can be filled by cartilage grafts. Alternatively, synthetic prostheses can be used. In our experience when primary closure was not possible, we have been using Goretex, Lactosorb, or artificial bone, usually with good results, but there is an increased risk of infection and the use of non-absorbable prosthesis in a developing thorax is a matter of concern.
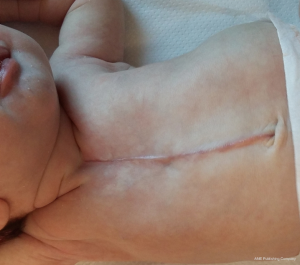
The outcome of the repair is usually good (Figure 4), in our 8 cases we had one partial recurrence in a patient in whom the prosthesis had to be removed due to infection. In this patient we were able to close the defect completely at 6 years of age by using a periosteal flap and multiple chondrotomies. At long term follow-up, we have observed in at least half of our patients the occurrence of a mild PE. If the PE had to be corrected (because of heart compression), we have repaired it through a Nuss procedure, with special care during retrosternal dissection, to avoid injuring the pericardium and myocardium. In these patients, if mediastinal adhesions make the retrosternal tunnel difficult to be created using a thoracoscopic approach, a digital dissection under view, though the same incision used for cleft repair can add safety to the procedure.
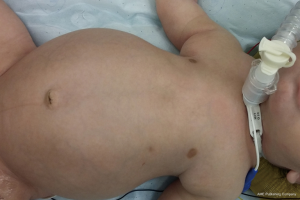
Jeune syndrome (ADT) (6)
ADT is a familial autosomal recessive disorder, presenting two variants of diverse severity. The key feature of ADT is the presence of short, horizontal and club-ended ribs and a low thoracic volume due to decreased thoracic diameters, with consequent insufficient lung ventilation (Figure 5). The more severe variant leads to fatal respiratory insufficiency during the first years of life and our presentation addresses the surgical treatment of this variant only. The indication to the surgery and its timing in ADT is determined by clinical factors such as repeated respiratory infections, respiratory distress, failure to thrive, pulmonary hypertension. Ideally, surgery has to be performed before cor pulmonale occurs, as this is a point of no return and the possibilities of success are very low. In severely affected infants, our approach includes a tracheostomy and home ventilation.
On the other hand, if the patient is relatively stable, it could be better to wait some months or in less severe cases a few years, as at this age the ribs will be more developed and will allow a better and easier correction.
Different approaches have been advocated for improving thoracic volumes: sternal split (16,17) vertical expandable titanium rib (18), and lateral thoracic expansion (LTE) (19). We have no experience of the first two techniques, so the latter will be the only surgical approach dealt with in this chapter.
LTE was described by Davis in 1995 (19): the lateral chest wall is exposed, the 4th and 9th ribs are split in the middle point, the 5th-6th rib and the 7th-8th rib are cut in a staggered fashion (alternatively posterior-anterior-posterior-anterior) and the stumps of the two adjacent ribs are approximated in the midline, so the result obtained will be two longer ribs out of 4 original short ribs. The operation is based not only on the expansion due to longer ribs, but also on repositioning the ribs in an oblique direction, instead of the horizontal direction of the ADT ribs. The new rib will be obtained from two rib stumps put together by titanium bars. The bars have special flanges at their ends that must be tightened around the ribs. Alternatively, they can be fixed by metallic screws. In both cases, it is important to free the anterior surface of the ribs from muscular and soft tissues so that the bar will adhere to the ribs in a stable way. For an easier and more secure fixation of the ribs, it would be better to operate on these babies possibly after 6-9 months of age. In our experience, we had to operate on infants earlier due to clinical conditions and though the ribs are really thin at this age, we were able to perform LTE.
In the original description of the technique by Davis (19), intercostal muscles and parietal pleura are divided in a staggered fashion in the opposite direction to the rib divisions. LTE was performed on both sides in two steps at least 6 months apart. During the first weeks after LTE it is important to maintain the patient well-ventilated to allow an adequate growth of the lung. For this purpose, mechanical ventilation through tracheostomy is helpful.
At the Gaslini Institute, 12 LTE have performed in 6 patients and we adopted some technical variations from the Davis technique. The most significant modification is to perform a bilateral approach as a single step. This approach, described also by other Authors (20), allows to expand both sides at the same time thus theoretically increasing the postoperative advantages of the LTE. We have observed that a longer intraoperative time was well tolerated by our patients. However, the post-operative period can be troublesome for some days or even weeks, so a bilateral approach should be attempted if a well-prepared intensive care used to treat severely affected neonates and infants is available.
Another modification to the Davis technique was performed in order to make the approach less invasive: in some case we have maintained a more superficial plane just below the posterior wall of the ribs, avoiding opening the pleura and without leaving a thoracic drain in the post-operative period. This approach could be criticized as the pleural cavity is not expanded thus preventing a complete LTE. In our opinion, however, LTE efficacy is mainly due to the reconfiguration of the ribs more than on the space obtained by opening the pleura. With a proper post-operative program of ventilation, we argue that the pleura will be stretched and expanded to the rib cage accordingly. Only longer follow ups and a larger series will tell if the original Davis approach or our less invasive procedure gives the better results.
It is very difficult to comment on the outcome of lateral expansion for ATD, as the series are small, and only few Centers in the world perform this procedure (21,22). In our experience we have 5 out of 6 children who survived, the oldest one being 11 years old. This patient was the only one who required an additional procedure to increase the chest wall stability with two more bars (one on each side), the others have not been operated on after LTE, but may require revision surgery at an older age.
In Davis’s experience, most of the patients improved their respiratory function at short and middle term (21), but we have no results on a long term basis.
Discussion
In pediatric age, the best surgical or conservative strategy to repair congenital chest wall anomalies is chosen according to a number of clinical factors and it requires an age specific approaches that take into account the natural history of the deformities, the progressive growth of the thoracic rib cage, as well as its malleability. A dedicated team with specific expertise is required to achieve the best possible results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Scanagatta) for the series “Pediatric Thoracic Surgery” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.08.04). The series “Pediatric Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional ethical approval was waived. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Currarino G, Silverman FN. Premature obliteration of the sternal sutures and pigeon-breast deformity. Radiology 1958;70:532-40. [Crossref] [PubMed]
- Poland A. Deficiency of the pectoralis muscles. Guys Hosp Rep 1841;6:191.
- Romanini MV, Torre M, Santi P, et al. Proposal of TBN classification of thoracic anomalies and treatment algorithm for Poland syndrome. Plast Reconstr Surg 2016;138:50-8. [Crossref] [PubMed]
- Romanini MV, Calevo MG, Puliti A, et al. Sem Pediatr Surg 2018;27:188-99. [Crossref]
- Torre M, Rapuzzi G, Carlucci M, et al. Phenotypic spectrum and management of sternal cleft: literature review and presentation of a new series. Eur J Cardiothorac Surg 2012;41:4-9. [Crossref] [PubMed]
- Jeune M, Carron R, Beraud C. Polychondrodystrophy with fatal thoracic blockage. Pediatrie 1954;9:390-2. [PubMed]
- Robicsek F. Congenital deformities of the anterior chest wall. In: Gellis SS, Kagan BM. editors. Current pediatric therapy. Philadelphia: W.B. Sanders, 1986;413-4.
- Ravitch MM. Unusual sternal deformity with cardiac symptoms operative correction. J Thorac Surg 1952;23:138-44. [PubMed]
- Robicsek F, Peeler BB. Minimally access open repair of pectus excavatum and carinatum (Robicsek Procedure). In: Saxena A. editor. Chest Wall deformities. Springer-Verlag Berlin Heidelberg, 2017;611-26.
- Welch KJ. Satisfactory surgical correction of pectus excavatum deformity in childhood: a limited opportunity. J Thorac Surg 1958;36:697-713. [PubMed]
- Fonkalsrud EW, Anselmo DM. Less extensive techniques for repair of pectus carinatum: the undertreated chest deformity. J Am Coll Surg 2004;198:898-905. [Crossref] [PubMed]
- Del Frari B, Schwabegger AH. Ten-year experience with the muscle split technique, bioabsorbable plates, and postoperative bracing for correction of pectus carinatum: the Innsbruck protocol. J Thorac Cardiovasc Surg 2011;141:1403-9. [Crossref] [PubMed]
- Brichon PY, Whilm JM. Correction of a severe pouter pigeon breast by triple sternal osteotomy with a novel titanium rib bridge fixation. Ann Thorac Surg 2010;90:e97-9. [Crossref] [PubMed]
- Baldelli I, Santi P, Dova L, et al. Body image disorders and surgical timing in patients affected by Poland syndrome: data analysis of 58 case studies. Plast Reconstr Surg 2016;137:1273-82. [Crossref] [PubMed]
- Torre M, Rapuzzi G, Giuda E. Thymectomy to achieve primary closure of total sterna cleft. J Pediatr Surg 2008;43:e17-20. [Crossref] [PubMed]
- Conroy E, Eustace N, McCormack D. Sternoplasty and rib distraction in neonatal Jeune syndrome. J Pediatr Orthop 2010;30:527-30. [Crossref] [PubMed]
- Phillips JD, van Aalst JA. Jeune’s syndrome (asphyxiating thoracic dystrophy): congenital and acquired. Semin Pediatr Surg 2008;17:167-72. [Crossref] [PubMed]
- Waldhausen JH, Redding GJ, Song KM. Vertical expandable prosthetic titanium rib for thoracic insufficiency syndrome: a new method to treat an old problem. J Pediatr Surg 2007;42:76-80. [Crossref] [PubMed]
- Davis JT, Ruberg RL, Lappink DM. Lateral thoracic expansion for Jeune’s asphyxiating dystrophy: a new approach. Ann Thorac Surg 1995;60:694-6. [Crossref] [PubMed]
- Muthialu N, Mussa S, Owens CM, et al. One-stage sequential bilateral thoracic expansion for asphyxiating thoracic dystrophy (Jeune Syndrome). Eur J Cardiothorac Surg 2014;46:643-7. [Crossref] [PubMed]
- Davis JT, Heistein JB, Castile RG, et al. Lateral thoracic expansion for Jeune’s syndrome: midterm results. Ann Thorac Surg 2001;72:872-7. [Crossref] [PubMed]
- de Vries J, Yntema JL, Van Die CE, et al. Jeune syndrome: description of 13 cases and a proposal for follow-up protocol. Eur J Pediatr 2010;169:77-88. [Crossref] [PubMed]
Cite this article as: Torre M, Palo F, Infante M. Open-surgery repair of congenital malformation of the chest: indications, technical tips and outcomes. Pediatr Med 2019;2:48.