
Bronchopulmonary dysplasia-associated pulmonary hypertension
Introduction
The term ‘bronchopulmonary dysplasia’ (BPD) was first used by Northway et al. to describe a chronic form of neonatal lung injury associated with barotrauma in preterm infants (1). Although there has been significant advancement in this field over the last couple of decades including sophisticated, newer and gentler methods of mechanical ventilation (MV), surfactant replacement therapy etc., the prevalence of BPD remains quite high. Classical or “old BPD”, present in the pre-surfactant era, was characterized by diffuse proliferative lung disease and postnatal lung injury. Jobe coined the term “new BPD” in 1999, to refer to the chronic lung disease of preterm infants at that time (2). This “new BPD” demonstrated much less alveolar septal fibrosis and airway damage when compared to its old counterpart, and was characterized with alveolar simplification and dysmorphic microvasculature (3). This dysmorphic growth and function of the pulmonary vasculature can cause pulmonary hypertension (PH) in infants with BPD. Bonikos et al. (4) first described cardiac atrial or ventricular stress, including cor pulmonale, in infants with BPD and since then it has been increasingly recognized in this population. A recent meta-analysis reported an accumulative estimated prevalence of PH in BPD to be 20%. The prevalence was estimated to be as high as 40% in severe BPD (5). BPD and BPD-associated PH (BPD-PH) have been correlated with devastating short- and long-term consequences for infants born prematurely. Retrospective studies demonstrate a 2-year morbidity rate of 26–47% in patients with BPD-PH (6,7). The pathophysiology of PH in BPD is not well understood at this time and there is a lack of standardized criteria for identifying preterm infants that are most at risk for developing BPD-PH. In this article we will review the pathophysiology, clinical presentation, diagnosis, suggested management and outcomes of infants with PH in the context of BPD.
Pathophysiology of BPD-PH
The pathogenesis of PH in BPD is affected by various maternal, genetic and environmental factors. Premature birth exposes the developing lung to an environment that can alter the normal developmental process and result in impaired lung development (8-10). Increased risk of BPD and PH has been shown in premature infants exposed to pre-eclampsia (11,12) and in infants with intrauterine growth restriction (13,14) due to disruption in vascular signaling pathways. Abnormal growth of the pulmonary circulation in BPD is characterized by decreased vascular branching, an altered pattern of vascular distribution within the lung interstitium, and persistent intrapulmonary venous anastomoses (15). Disruption in vascular growth results in decreased vessel density throughout the pulmonary microcapillary network (16). This leads to decreased cross sectional area for pulmonary blood flow and increased pulmonary vascular resistance, which in turn alters pulmonary vasoreactivity and causes vascular remodeling. This dysregulated pulmonary vasculature has overall less area for gaseous exchange. In patients with BPD who have airway and parenchymal injury and inflammation, this results in hypoxic vasoconstriction and impaired pulmonary blood flow, especially under stress (17). Chronic hypoxia also contributes to pulmonary arterial vasoreactivity, leading to vascular remodeling with intimal hyperplasia and increased muscularization of small pulmonary arteries. Endothelial injury due to environmental stressors induces smooth muscle cell proliferation in the media of the small pulmonary arteries, precocious maturation of immature mesenchymal cells into mature smooth muscle cells and incorporates fibroblasts into the vessel wall. Such pulmonary vascular remodeling, along with failure of the lung vasculature to catch up with the normal term lung, may contribute to the pulmonary vessel disease associated with BPD (18).
While the lung pathology of infants with PH and BPD has many common elements, it is important to note that not all infants with BPD develop PH; it is more commonly seen in infants with severe BPD (6). The index of suspicion for diagnosing PH is also higher in this subgroup and they are screened more frequently for PH. Infants with other associated conditions like large patent ductus arteriosus, pulmonary vein stenosis (PVS) or aorto-pulmonary collaterals are more at risk for developing severe BPD and PH (7). Infants with lower baseline oxygen saturations or frequent hypoxic episodes are also at higher risk for developing PH due to recurrent episodes of hypoxia and increased vasoreactivity. Maternal chorioamnionitis and postnatal lung infections also have a negative influence on lung development and may lead to abnormal lung vasculature growth (19). Lastly, BPD has a strong genetic predisposition (8), and so it can be postulated that development of PH in BPD also has some genetic influence (20,21). A proposed schema of the interacting factors involved in the pathogenesis of BPD-PH is illustrated in Figure 1.
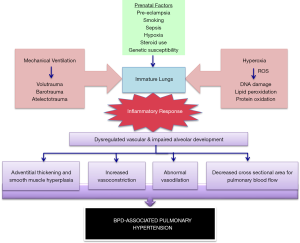
Clinical features
Infants with BPD who develop PH may be asymptomatic or may present with increased need for respiratory support, failure to wean from respiratory support, frequent hypoxic events and/or impaired somatic growth due to increased metabolic demands. Many institutions screen infants with BPD for PH. Asymptomatic infants may be diagnosed as a result of such screening echocardiograms or incidentally while looking for other pathology. Symptomatic infants may have sudden deterioration in their clinical status and have a pulmonary hypertensive crisis. Such episodes may be idiopathic in origin or may be precipitated by infection (systemic sepsis, respiratory or urinary tract infections), aspiration events or changes in lung pathology (atelectasis, mucous plugging, etc.). On the other hand, some infants may have gradual deterioration in their clinical status and have a more insidious onset. They may initially present with a gradual increase in oxygen requirement and/or ventilator support and may progress to develop right heart dysfunction (19). It is important to have a high index of suspicion for PH in these infants so as to be able to timely diagnose them and adequately manage this disease.
Screening and diagnosis of BPD-PH
The Pediatric Task Force at the 6th World Symposium on PH suggested a recent update to the definition of pediatric PH, where they now suggest including patients with mean pulmonary artery pressure >20 mmHg and pulmonary vascular resistance ≥3 Wood units (WU) to identify pre-capillary PH (22). Most neonatal intensive care units use a clinical algorithm for the identification and evaluation of infants at risk for BPD-PH. However, adoption of a standardized clinical algorithm is a challenge given the lack of agreed-upon definition of PH in this setting and inadequacy of echocardiography to detect PH, which is the most commonly used screening tool. In 2015, the American Heart Association (AHA) and American Thoracic Society (ATS) published recommendations for the care of patients with pediatric PH. These guidelines provided the initial framework for screening and care of infants with BPD-PH (23). In 2017, the Pediatric Pulmonary Hypertension Network (PPHNet), a multi-disciplinary panel of PH experts published more detailed guidelines that provide practical clinical recommendations for the evaluation, diagnosis and management of infants with BPD-PH (24).
These guidelines suggest obtaining an echocardiogram for premature infants with severe hypoxic respiratory failure with minimal parenchymal disease and those that still need MV at 7 days or more in order to identify infants with acute PH that might benefit from therapy. The guidelines also suggest getting an echocardiographic evaluation for infants with diagnosis of mild or moderate BPD (at 36 weeks postmenstrual age) and those with sustained need for significant respiratory support at any age. They suggest monitoring infants with mild BPD clinically, with echocardiography to be obtained only if they clinically deteriorate (24). At our institution, we screen all patients with the diagnosis of BPD for PH, irrespective of the severity of their disease. Figure 2 illustrates the protocol for screening infants with BPD for PH used at St. Christopher’s Hospital for Children, Philadelphia, PA, USA. The most commonly used modalities in the screening and diagnosis of PH are discussed below.
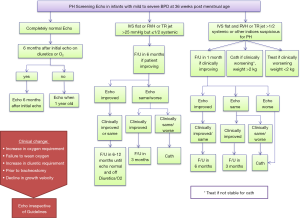
Echocardiogram
This is the most commonly used screening tool for PH in infants with BPD. However, the echocardiogram uses somewhat subjective criteria for diagnosis, and it does not have great inter-rater reliability. The PPHNet guidelines suggest that a complete echocardiogram for PH screening in preterm infants should include, at a minimum: (I) a complete anatomic evaluation, to identify and characterize the physiologic contribution of structural abnormalities, shunts and pulmonary veins; (II) assessment of right and left ventricular size, hypertrophy; systolic and diastolic function; (III) systolic and diastolic interventricular septal position; (IV) tricuspid regurgitation jet velocity (TRJV) when present; and (V) documentation of the systemic blood pressure at the time of the study (24). Although TRJV is the most frequently used measurement for evaluating PH, many infants do not have a detectable TRJ at the time of the study and may also be affected by the lung inflation (25). In a study done to evaluate clinical utility of echocardiograms in the diagnosis of PH in infants with chronic lung disease, Mourani et al. found that when compared with cardiac catheterization measurements, echocardiographic estimates of systolic pulmonary artery pressure correctly diagnosed the presence or absence of PH in 79% of the studies but determined the severity of PH (severe PH was defined as pulmonary/systemic pressure ratio of ≥0.67) correctly in only 47% of those studies. In the absence of a measurable TRJV, qualitative echocardiographic findings, including right atrial enlargement, right ventricular hypertrophy, right ventricular dilation, pulmonary artery dilation, and septal flattening, either alone or in combination, have relatively poor predictive value (26).
A growing body of evidence also suggests that acquired PVS can results in obstructed pulmonary blood flow and contribute to increased pulmonary pressures. A recent study of 213 infants with severe BPD demonstrated the presence of PVS in 5% of these infants and these infants were noted to have worse outcomes with higher mortality (27). Although echocardiography may be able to identify most of these cases, cardiac catheterization or detailed imaging may be required for better evaluation of the vessel involvement.
Cardiac catheterization
Cardiac catheterization is the gold standard method for diagnosing PH but is a much more invasive test and has been reserved for patients for whom noninvasive assessments are considered inadequate and for assessment of acute vasoreactivity, to guide long-term therapy. The PPHNet recommendations suggest obtaining a cardiac catheterization prior to initiating pulmonary vasodilator therapy but the procedure is often deferred by the patient care teams because of the risk for significant vascular and hemodynamic complications in this group of very small infants. If obtained, cardiac catheterization can assist in defining the reversible component of vasoconstriction with therapy and detect PVS, aortopulmonary collaterals and left heart dysfunction (25). Despite its many advantages, cardiac catheterization involves many risks in this population, including the need for sedation and intubation as well as additional complications from hemodynamic instability, vascular events and thrombosis. Cardiac catheterization in infants with PH is associated with 6% risk of death or resuscitation (28). Hence it is suggested that cardiac catheterization be attempted with caution and should be reserved for management of infants where it is deemed absolutely necessary for treatment optimization.
Biomarkers
Currently there is no single reliable biomarker that can diagnose, predict severity and monitor response to therapy. Serum brain natriuretic peptide (BNP) and N-terminal-pro-brain natriuretic peptide (NT-proBNP), which are released by the myocardium in response to stretch, are the most commonly used biomarkers for monitoring changes in disease status in infants with known PH, but are not specific for right ventricular stress or PH. The PPHNet guidelines suggest using serial BNP or NT-proBNP levels to monitor disease progression/regression, response to therapy, in conjunction with echocardiography rather than in isolation (24). Various other biomarkers are currently being explored in the setting of BPD that can be used to identify the infants most at risk for developing severe disease and/or PH. Identification of such novel biomarkers will potentially greatly facilitate the diagnosis and management of these infants (29).
Management
The evaluation and management of infants with BPD-PH is an area of immense debate and the practices vary greatly between individuals and institutions. The current evidence suggests that there are significant comorbidities present in infants with this diagnosis. Hence, they should undergo a comprehensive evaluation of the parenchymal lung and large airway disease prior to initiation for therapy for PH. This should include evaluation for hypoxemia, gastroesophageal reflux, aspiration, vocal cord abnormalities, subglottic stenosis, laryngo-bronchomalacia, pulmonary stenosis, cardiac dysfunction and other cardiac anomalies. Oxygen therapy should be optimized with a goal to keep target saturations between 92–95% to prevent the adverse effects of hypoxia without causing additional lung injury by hyperoxia (24).
The suggested threshold for initiation of vasodilators includes TRJV >3 m/second, estimated right ventricle/left ventricle systolic pressure >0.5, and septal flattening in absence of a significant left to right shunt. For cardiac catheterization significant measurements would include a ratio of pulmonary artery to systemic pressure ≥0.5, indexed pulmonary venous resistance ≥3 WU, or pulmonary/systemic resistance (Rp/Rs) ≥0.5, and a normal wedge or left ventricle end diastolic pressure without evidence of significant PVS. The various agents used in the treatment of PH in BPD are discussed below.
Management of acute hypertensive crisis and use of nitric oxide (NO)
Acute exacerbations of PH occur in infants with BPD, e.g., due to viral infections or mechanical airway obstruction. These infants can have a labile respiratory status and have profound hypoxemia with hypotension in response to routine daily care interventions. An initial evaluation should be performed with a chest X ray to rule out atelectasis, pneumonia and/or other parenchymal lung pathologies. Ventilator support must be optimized, and treatment of the underlying cause should be done—including antibiotics for infection if warranted, treatment of gastroesophageal reflux and steroids for inflammation, if needed (24). Once optimized, a dose of 10–20 ppm of inhaled NO (iNO) should be initiated for the acute PH crisis. Once the infant is stabilized and improving, iNO should be weaned initially aggressively to 5 ppm and then gradually by 1 ppm at selected intervals based on the infant’s tolerance. NO produced by endothelial cells stimulate soluble guanylate cyclase to increase cyclic guanosine monophosphate (cGMP), which causes relaxation of the vascular smooth muscle cell. iNO acts as a potent pulmonary vasodilator by acting on the same pathway (30). iNO has been shown to potentiate the vasodilator response of oxygen and a combination of iNO and supplemental oxygen can often help to reduce the pulmonary vascular resistance (31). Because of its short half-life, iNO has to be administered continuously and its use is restricted to the inpatient setting. Addition of a second agent like sildenafil should be considered if the response with iNO is insufficient, delivery of iNO is logistically difficult or if weaning of iNO is a problem. Phospodiesterase-5 (PDE-5) inhibitors like sildenafil cause vasodilation by increasing the activity of cGMP and can potentiate the effect of iNO or prevent rebound PH after discontinuing iNO (18).
Pharmacotherapy
PH present in BPD consists of 2 components—a fixed component, due to decreased cross sectional area of pulmonary vasculature in the setting of arrested lung development and remodeling of pulmonary arteries; and a responsive component presumably due to pulmonary vascular smooth muscle, which responds to vasodilators. The mainstay of management has been optimization of BPD management to promote lung development and use of pulmonary vasodilators. The therapeutic agents used are either vasodilators that act on the NO pathway or prostacyclin pathway, or inhibitors of vasoconstrictors that modulate the endothelin pathway (30). These drugs have been summarized in Table 1.
Table 1
| Name of drug | Formulations available | Dose | Adverse effects |
|---|---|---|---|
| Sildenafil (phosphodiesterase-5 inhibitor) | Oral: tablets and suspension; intravenous | PO: 1 mg/kg/dose q 6–8 h; start with low dose (0.3–0.5 mg/kg/dose) and gradually increase to 1 mg/kg/dose q 8 h as tolerated; intravenous: 0.25–0.5 mg/kg/dose q 6–8 h | Systemic hypotension, irritability, bronchospasm, rarely priapism |
| Milrinone (phosphodiesterase-3 inhibitor) | Intravenous continuous infusion | 0.15–0.5 mcg/kg/min | Systemic hypotension, arrhythmia, risk of decreased myocardial perfusion |
| Bosentan (endothelin receptor antagonist) | Oral dispersible tablets | 0.5–1 mg/kg BID, may increase up to 2 mg/kg BID in 2–4 weeks | Liver dysfunction (monitor liver function tests monthly), VQ mismatch, hypotension, anemia, known teratogen (precautions for pregnant caregiver) |
| Epoprostenol (Flolan) | Intravenous | Start at 1–2 ng/kg/min, titrate up slowly every 4–6 h to 20 ng/kg/min | Hypotension, VQ mismatch, GI disturbances, tachyphylaxis and risk of rebound PH |
| Iloprost | Inhaled | 2.5–5 mcg every 2–4 h. Can be given as continuous inhalation during mechanical ventilation | Bronchospasm, hypotension, pulmonary hemorrhage, ventilator tube blocking due to crystallization, GI disturbances |
| Treprostinil | Intravenous; subcutaneous | Start at 2 ng/kg/min and titrate every 4–6 h up to 20 ng/kg/min | Similar to epoprostenol. Subcutaneous form: local site pain |
BPD-PH, bronchopulmonary associated-pulmonary hypertension; PO, per os; VQ, ventilation-perfusion; GI, gastrointestinal.
Drugs acting on the NO pathway
Sildenafil
cGMP is an intracellular mediator that controls vascular contractility via NO activity. Increased concentration of PDE-5 isoenzyme causes degradation of cGMP through hydrolysis. Sildenafil acts by inhibiting the PDE-5 and increasing levels of cGMP, thereby augmenting vasodilation. In 2005 the Food and Drug Administration (FDA) approved the use of sildenafil for PH in adults and it has been widely used off-label for PH in neonates due to its ease of administration compared to other PH medications. Despite widespread use, there is minimal data supporting its efficacy in treating PH in neonates and infants (32,33). In 2012 the FDA issued a warning against the use of sildenafil in children between 1–17 years of age based on the data from the STARTS-2 trial that showed a high mortality in older children taking higher dose of sildenafil (>3 mg/kg/day) (34). In a retrospective cohort study of 269 pediatric patients with PH (50% of which had BPD-PH), use of low dose sildenafil (2 mg/kg/day or less) was well tolerated and was found to be safe with an acceptable side effect profile. Infant with BPD-PH were most likely to show improvement in PH, allowing for discontinuation of sildenafil in 45% of patients in that category (35). Tadalafil is a longer acting PDE5 inhibitor that has been approved for management of adult PH, but its use and efficacy in pediatric PH is still not known.
Milrinone
Milrinone is a PDE-3 inhibitor that increases cyclic adenosine monophosphate (cAMP) by inhibiting its breakdown in the arterial smooth muscle cells and myocardium. By increasing cAMP in cardiac muscle and vascular cells, it causes relaxation of vascular smooth muscle, enhances myocardial contractility (inotropy) and improves myocardial relaxation (lusitropy) (36). Hence it improves cardiac function both directly and by reducing afterload. It can also be used as an adjuvant to iNO to promote pulmonary vasodilation (37). Since it can only be used as a continuous infusion, its use is limited for hospitalized infants with acute exacerbations.
Drugs acting of the endothelin pathway
Bosentan
Endothelin-1 (ET-1) is a potent vasoconstrictor that is produced in response to hypoxia. It promotes smooth muscle proliferation, endothelial cell dysfunction, inflammation and fibrosis (37). ET-1 acts via two G-protein coupled receptors—ETA that occurs on vascular smooth muscle cells, and ETB that is present on endothelial and vascular smooth muscle cells. ET-1 is strongly implicated in the pathogenesis of PH in various clinical and preclinical studies (38,39). Bosentan is an antagonist of both ETA and ETB receptors (with a slightly higher affinity for ETA) and is used extensively in adults with PH (39). In 2017, bosentan was approved for use by the FDA for PH in children 3 years of age or older and its experience in pediatric patients has been favorable with improved outcomes (40). There is limited data available for its use in patients with BPD-PH; however, its use is increasingly been seen in this population, especially as maintenance therapy for PH (41).
Other endothelin receptor antagonists include ambrisentan, which is a selective antagonist of ETA and macitentan, which antagonizes both ETA and ETB receptors. However, there is no published data regarding their use in infants with BPD-PH.
Drugs acting of the prostacyclin pathway
Prostacyclin (PGI2) is an arachidonic acid metabolite and is produced endogenously by the vascular endothelium. It mediates vasodilation by activating adenylate cyclase and increasing cAMP in the pulmonary arterial smooth muscle cell (25). Several different PGI2 analogs have been used in adults and children with PH and are discussed below.
Epoprostenol
The FDA approved this drug in 1995 for treatment of severe chronic PH in adults. Since its half-life is very short (~6 minutes) it must be delivered continuously via a dedicated central line with caution as any unplanned interruption in delivery may be dangerous (42,43). To limit the systemic side effects, the inhaled form of epoprostenol is commonly used in adult patients but there is limited experience of its use in preterm patients (25). It has however been shown to improve oxygenation in term infants with primary PH of newborn (PPHN), without significant adverse effects (44).
Iloprost
Iloprost has a half-life of 20–30 minutes and is a more stable compound and so can be delivered by continuous inhalation to limit the systemic side effects (45). There is limited data from case series and case reports that suggest improved oxygenation in infants with BPD-PH with use of iloprost either alone or in combination with sildenafil (46,47). It has also shown promise in improving oxygenation in the setting of PPHN (48,49).
Treprostinil
It is a stable PGI2 analogue that can be delivered by intravenous or subcutaneous route. Continuous subcutaneous delivery can be used to transition children who were previously stable on continuous IV epoprostenol (50). Limited experience with the use of this drug in the setting of BPD-PH shows improvement in right ventricular function and PH with serial echocardiograms and decreased respiratory support within weeks of therapy initiation without significant side effects (51,52).
New and emerging drugs—prostacyclin receptor agonists
Selexipag
It is an oral, long acting, highly selective prostacyclin receptor agonist that causes vasodilation and inhibits smooth muscle cell proliferation. It has been used in adults as oral triple combination therapy (with ET receptor antagonist and PDE inhibitor) in adults with severe PH. However, data on its use in pediatric population is limited, with the first off-label use reported in 2017 (53,54). Since then there have been limited data describing its use in children ranging in age from 1.5 to 17 years with idiopathic pulmonary arterial hypertension or PH with congenital heart disease (55). Its use has not been reported in the setting of BPD-PH.
Beraprost
It was the first oral prostacyclin receptor agonist. However, it was not approved by FDA in the United States due to its adverse effects but is approved for use in Japan and South Korea in patients with PH (56).
A suggested sequence of these of these drugs is to initiate with iNO at 10–20 ppm. Obtain an echocardiogram and monitor infant for change in clinical status. If clinical status remains unchanged or worsens, sildenafil should be added. Sildenafil can also be used for chronic maintenance therapy after iNO has been weaned off. Treatment with prostacyclin derivatives or ET-1 antagonists should be considered in patients unresponsive to the above treatment. Echocardiogram should be obtained when changing the treatment modality to evaluate the response to therapy. Cardiac catheterization should be considered if first- and second-line treatments are ineffective and patient is stable. Serial BNP or NT-proBNP levels may be obtained weekly or twice weekly in unstable patients to assess response to therapy (24,30).
Outcomes of BPD-PH
The mortality rate in infants with PH is high, especially in infants with severe PH. In a retrospective study evaluating outcomes of 36 patients with BPD and severe/moderate PH, follow‐up at 35 months showed improvement in PH in 15 patients after treatment; persistent severe PH in 4 patients despite treatment and closure of shunts; spontaneous improvement in 4 patients who were not on treatment; while 8 patients (26%) had died (7). In another study, evaluating 42 premature infants with BPD and PH, estimated survival rates were 64%±8% at 6 months and 53%±11% at 2 years after diagnosis of pulmonary artery hypertension. Severe PH and small for gestational age were associated with worse survival rates in this study. Among 26 survivors, improvement in PH was observed in 24 patients (92%) (6). Presence of PVS has also been shown to increase mortality in infants with BPD-PH. Among infants with severe BPD-PH, survival was lower among infants with PVS than those without PVS [5/9 (56%] vs. 26/30 (87%); P=0.01] (27). Lung transplantation is a therapeutic option for infants and children with severe PH unresponsive to therapy. However it is reserved for patients who do not have other comorbidities. As such, children with severe BPD-PH are not good candidates for this treatment since many of them have severe comorbidities associated with prematurity.
Conclusions
BPD-PH is a complex disease with immense variability in its clinical phenotype. Although we have come a long way in understanding its pathophysiology, clinical evidence is still needed for the use of novel biomarkers in the diagnosis and management and to test the efficacy and safety of various therapeutic agents. Routine screening of infants with BPD is important for the early detection of BPD-PH. Using a multidisciplinary approach to optimize the management of infants with BPD will also help to prevent the short- and long-term morbidities associated with BPD-PH.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.02.01). VB serves as an unpaid editorial board member of Pediatric Medicine from Aug 2018 to Jul 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967;276:357-68. [Crossref] [PubMed]
- Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999;46:641-3. [Crossref] [PubMed]
- Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710-7. [Crossref] [PubMed]
- Bonikos DS, Bensch KG, Northway WH Jr, et al. Bronchopulmonary dysplasia: the pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Hum Pathol 1976;7:643-66. [Crossref] [PubMed]
- Arjaans S, Zwart EAH, Ploegstra MJ, et al. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: A systematic review and meta-analysis. Paediatr Perinat Epidemiol 2018;32:258-67. [Crossref] [PubMed]
- Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120:1260-9. [Crossref] [PubMed]
- del Cerro MJ, Sabate Rotes A, Carton A, et al. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol 2014;49:49-59. [Crossref] [PubMed]
- Bhandari V, Gruen JR. The genetics of bronchopulmonary dysplasia. Semin Perinatol 2006;30:185-91. [Crossref] [PubMed]
- De Paepe ME. Pathology of Bronchopulmonary Dysplasia. In: Bhandari V. editor. Bronchopulmonary Dysplasia. Cham: Springer International Publishing, 2016:149-64.
- Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol 2006;30:179-84. [Crossref] [PubMed]
- Tang JR, Karumanchi SA, Seedorf G, et al. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2012;302:L36-46. [Crossref] [PubMed]
- Foidart JM, Schaaps JP, Chantraine F, et al. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia--a step forward but not the definitive answer. J Reprod Immunol 2009;82:106-11. [Crossref] [PubMed]
- Rozance PJ, Seedorf GJ, Brown A, et al. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am J Physiol Lung Cell Mol Physiol 2011;301:L860-71. [Crossref] [PubMed]
- Check J, Gotteiner N, Liu X, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol 2013;33:553-7. [Crossref] [PubMed]
- Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Fetal Neonatal Med 2003;8:73-81. [Crossref] [PubMed]
- Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600-7. [Crossref] [PubMed]
- Baker CD, Abman SH, Mourani PM. Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Pediatr Allergy Immunol Pulmonol 2014;27:8-16. [Crossref] [PubMed]
- Mourani PM, Abman SH. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clin Perinatol 2015;42:839-55. [Crossref] [PubMed]
- Ambalavanan N, Mourani P. Pulmonary hypertension in bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 2014;100:240-6. [Crossref] [PubMed]
- Trittmann JK, Gastier-Foster JM, Zmuda EJ, et al. A single nucleotide polymorphism in the dimethylarginine dimethylaminohydrolase gene is associated with lower risk of pulmonary hypertension in bronchopulmonary dysplasia. Acta Paediatr 2016;105:e170-5. [Crossref] [PubMed]
- Trittmann JK, Jin Y, Chicoine LG, et al. An arginase-1 SNP that protects against the development of pulmonary hypertension in bronchopulmonary dysplasia enhances NO-mediated apoptosis in lymphocytes. Physiol Rep 2016;4: [Crossref] [PubMed]
- Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 2019;53: [Crossref] [PubMed]
- Abman SH, Hansmann G, Archer SL, et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation 2015;132:2037-99. [Crossref] [PubMed]
- Krishnan U, Feinstein JA, Adatia I, et al. Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. J Pediatr 2017;188:24-34.e1. [Crossref] [PubMed]
- Berkelhamer SK, Mestan KK, Steinhorn R. An update on the diagnosis and management of bronchopulmonary dysplasia (BPD)-associated pulmonary hypertension. Semin Perinatol 2018;42:432-43. [Crossref] [PubMed]
- Mourani PM, Sontag MK, Younoszai A, et al. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008;121:317-25. [Crossref] [PubMed]
- Swier NL, Richards B, Cua CL, et al. Pulmonary Vein Stenosis in Neonates with Severe Bronchopulmonary Dysplasia. Am J Perinatol 2016;33:671-7. [Crossref] [PubMed]
- Taylor CJ, Derrick G, McEwan A, et al. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth 2007;98:657-61. [Crossref] [PubMed]
- Piersigilli F, Bhandari V. Biomarkers in neonatology: the new "omics" of bronchopulmonary dysplasia. J Matern Fetal Neonatal Med 2016;29:1758-64. [PubMed]
- Lal CV, Ambalavanan N. Pulmonary Hypertension in Bronchopulmonary Dysplasia. In: Bhandari V. editor. Bronchopulmonary Dysplasia. Cham: Springer International Publishing, 2016:259-79.
- Mourani PM, Ivy DD, Gao D, et al. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2004;170:1006-13. [Crossref] [PubMed]
- Mourani PM, Sontag MK, Ivy DD, et al. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr 2009;154:379-84, 384.e1-2.
- Kelly LE, Ohlsson A, Shah PS. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst Rev 2017;8:CD005494 [PubMed]
- Barst RJ, Beghetti M, Pulido T, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation 2014;129:1914-23. [Crossref] [PubMed]
- Cohen JL, Nees SN, Valencia GA, et al. Sildenafil Use in Children with Pulmonary Hypertension. J Pediatr 2019;205:29-34.e1. [Crossref] [PubMed]
- Silver PJ, Harris AL, Canniff PC, et al. Phosphodiesterase isozyme inhibition, activation of the cAMP system, and positive inotropy mediated by milrinone in isolated guinea pig cardiac muscle. J Cardiovasc Pharmacol 1989;13:530-40. [Crossref] [PubMed]
- Lakshminrusimha S, Mathew B, Leach CL. Pharmacologic strategies in neonatal pulmonary hypertension other than nitric oxide. Semin Perinatol 2016;40:160-73. [Crossref] [PubMed]
- Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993;328:1732-9. [Crossref] [PubMed]
- Abman SH. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu Rev Med 2009;60:13-23. [Crossref] [PubMed]
- Maiya S, Hislop AA, Flynn Y, et al. Response to bosentan in children with pulmonary hypertension. Heart 2006;92:664-70. [Crossref] [PubMed]
- Krishnan U, Krishnan S, Gewitz M. Treatment of pulmonary hypertension in children with chronic lung disease with newer oral therapies. Pediatr Cardiol 2008;29:1082-6. [Crossref] [PubMed]
- Barst RJ. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin. Heart 1997;77:299-301. [Crossref] [PubMed]
- Zaidi AN, Dettorre MD, Ceneviva GD, et al. Epoprostenol and home mechanical ventilation for pulmonary hypertension associated with chronic lung disease. Pediatr Pulmonol 2005;40:265-9. [Crossref] [PubMed]
- Berger-Caron F, Piedboeuf B, Morissette G, et al. Inhaled Epoprostenol for Pulmonary Hypertension Treatment in Neonates: A 12-Year Experience. Am J Perinatol 2018; [Epub ahead of print]. [PubMed]
- Ewert R, Schaper C, Halank M, et al. Inhalative iloprost - pharmacology and clinical application. Expert Opin Pharmacother 2009;10:2195-207. [Crossref] [PubMed]
- Gurakan B, Kayiran P, Ozturk N, et al. Therapeutic combination of sildenafil and iloprost in a preterm neonate with pulmonary hypertension. Pediatr Pulmonol 2011;46:617-20. [Crossref] [PubMed]
- Piastra M, De Luca D, De Carolis MP, et al. Nebulized iloprost and noninvasive respiratory support for impending hypoxaemic respiratory failure in formerly preterm infants: a case series. Pediatr Pulmonol 2012;47:757-62. [Crossref] [PubMed]
- Kahveci H, Yilmaz O, Avsar UZ, et al. Oral sildenafil and inhaled iloprost in the treatment of pulmonary hypertension of the newborn. Pediatr Pulmonol 2014;49:1205-13. [Crossref] [PubMed]
- Janjindamai W, Thatrimontrichai A, Maneenil G, et al. Effectiveness and safety of intravenous iloprost for severe persistent pulmonary hypertension of the newborn. Indian Pediatr 2013;50:934-8. [Crossref] [PubMed]
- Ivy DD, Claussen L, Doran A. Transition of stable pediatric patients with pulmonary arterial hypertension from intravenous epoprostenol to intravenous treprostinil. Am J Cardiol 2007;99:696-8. [Crossref] [PubMed]
- Ferdman DJ, Rosenzweig EB, Zuckerman WA, et al. Subcutaneous treprostinil for pulmonary hypertension in chronic lung disease of infancy. Pediatrics 2014;134:e274-8. [Crossref] [PubMed]
- Krishnan U, Takatsuki S, Ivy DD, et al. Effectiveness and safety of inhaled treprostinil for the treatment of pulmonary arterial hypertension in children. Am J Cardiol 2012;110:1704-9. [Crossref] [PubMed]
- Geerdink LM, Bertram H, Hansmann G. First-in-child use of the oral selective prostacyclin IP receptor agonist selexipag in pulmonary arterial hypertension. Pulm Circ 2017;7:551-4. [Crossref] [PubMed]
- Lang IM, Gaine SP. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev 2015;24:630-41. [Crossref] [PubMed]
- Koestenberger M, Hansmann G. Should we use the oral selective IP receptor agonist selexipag off-label in children with pulmonary arterial hypertension? Pulm Circ 2018;8:2045894018793580 [Crossref] [PubMed]
- Pugliese SC, Bull TM. Clinical use of extended-release oral treprostinil in the treatment of pulmonary arterial hypertension. Integr Blood Press Control 2016;9:1-7. [PubMed]
Cite this article as: Sahni M, Bhandari V. Bronchopulmonary dysplasia-associated pulmonary hypertension. Pediatr Med 2019;2:4.

